Quality control product of chromosome aneuploidy detection kit capable of being stably stored and application thereof
A detection kit, aneuploidy technology, applied in the direction of microbial determination/inspection, biochemical equipment and methods, etc., can solve the problem of not meeting the requirements for the validity period of quality control products, nucleic acid degradation, etc., to achieve inhibition of DNA degradation, The effect of easy storage and extended expiration date
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
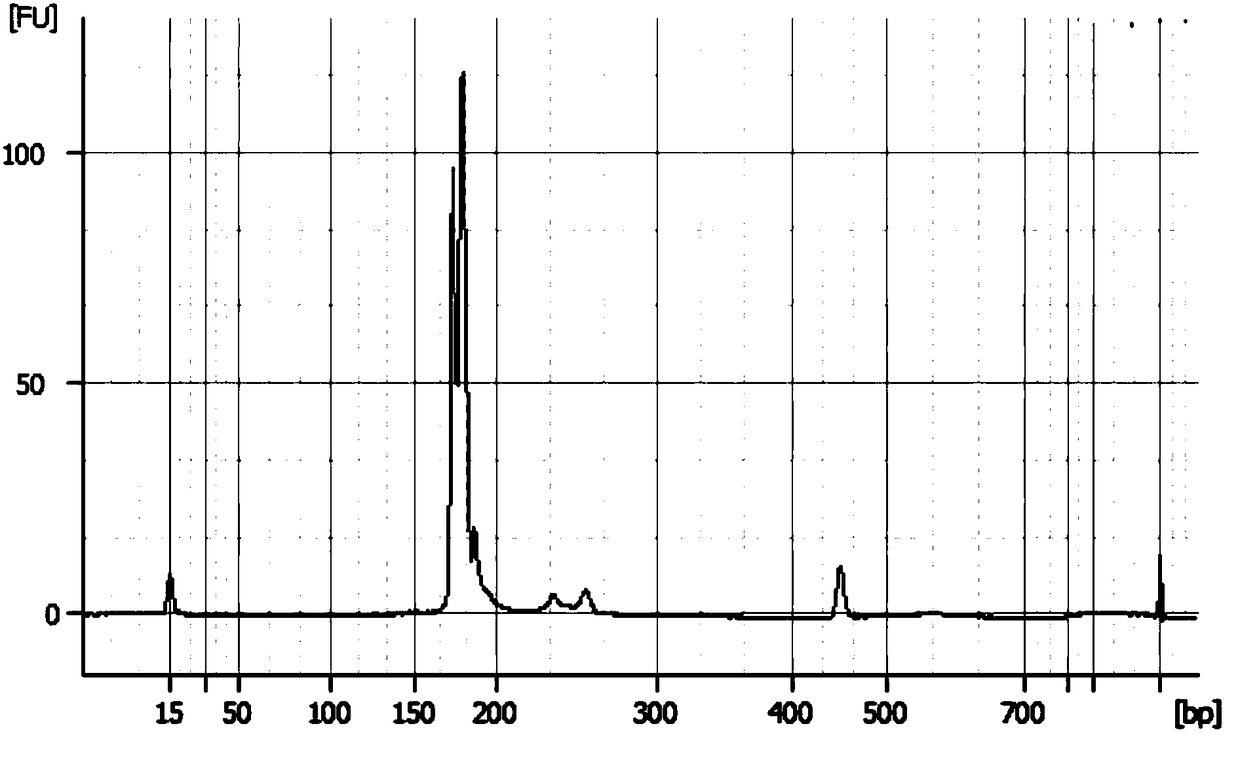
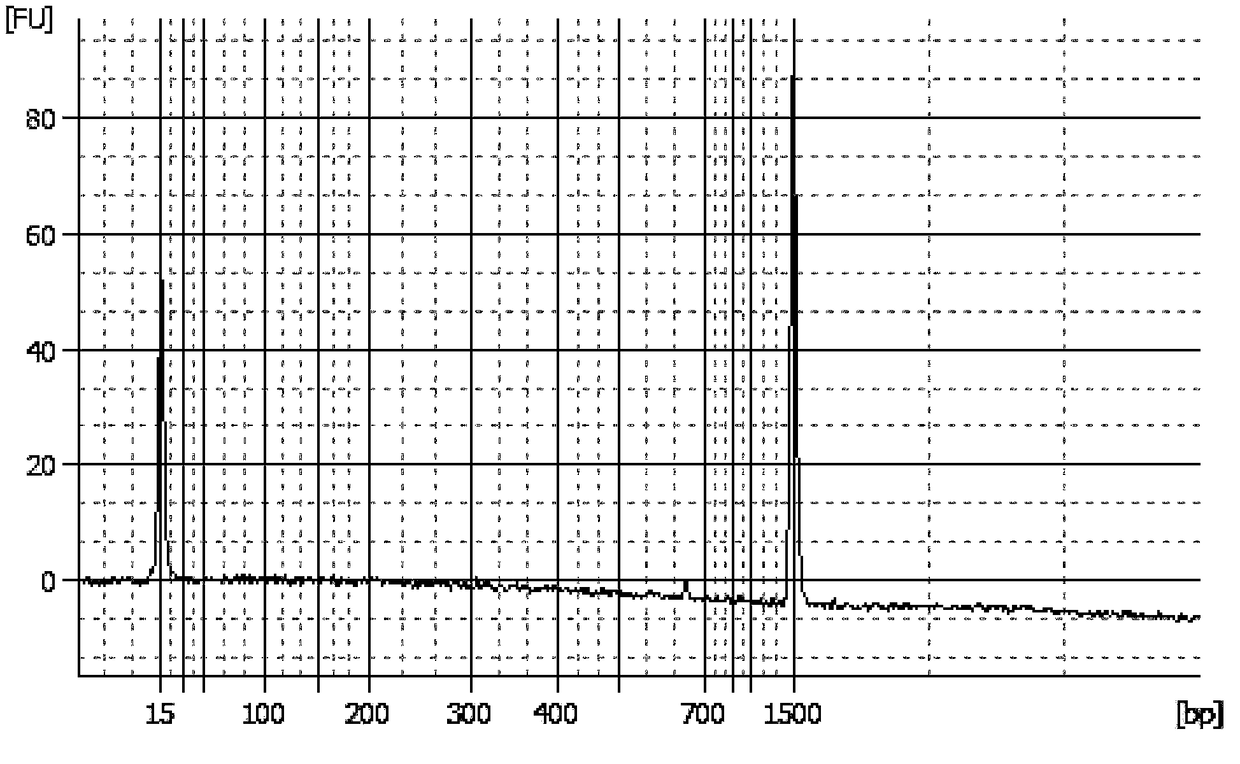
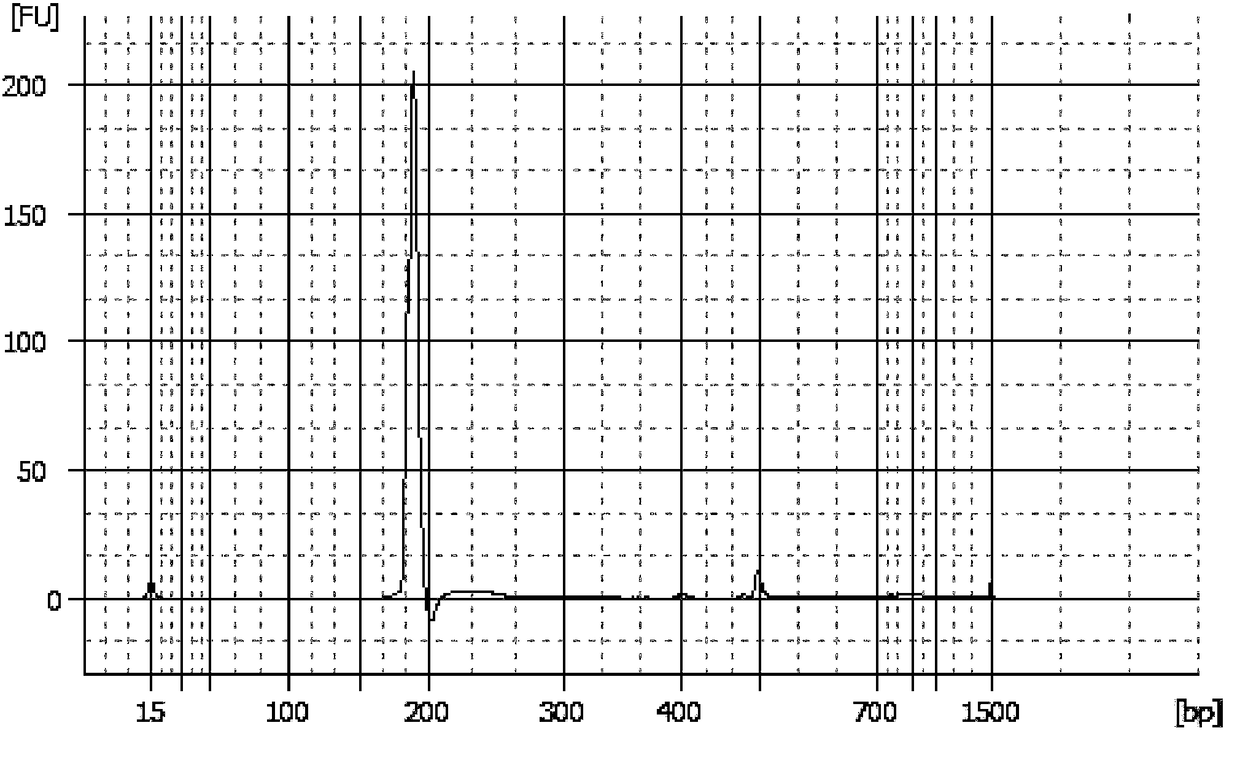
[0033] Embodiment 1, the quality control product of the chromosomal aneuploidy (T21, T18, T13) detection kit that can be stored stably and its preparation
[0034]This example provides a complete set of quality control materials for a stable chromosomal aneuploidy (T21, T18, T13) detection kit, including not only positive reference materials, negative reference materials, detection limit reference materials, but also microdeletion micro Duplicate references, chimera references, duplicate references.
[0035] 1. Positive reference product and its preparation
[0036] The positive reference substance is used for the positive detection rate of the quality control kit to be quality controlled, and its preparation method comprises the following steps:
[0037] ① DNA extraction: Select and extract the T21 human genomic DNA with a karyotype of 47, the T18 human genomic DNA with a karyotype of 47, and the T13 human genomic DNA with a karyotype of 47; this step can be performed us...
Embodiment 2
[0083] Embodiment 2 is a modification example of Embodiment 1. The only difference is that the concentrations of aurintricarboxylate ammonium salt, glycine, and bestatin hydrochloride contained in each reference product of the quality control product are respectively 100 μm, 80 mM, and 20 nM.
Embodiment 3
[0084] Embodiment 3 is a modification example of Embodiment 1. The only difference is that the concentrations of aurintricarboxylate ammonium salt, glycine, and bestatin hydrochloride contained in each reference product of the quality control product are 500 μm, 180 mM, and 60 nM, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com