Disposable auxiliary device and method for preparing radioactive medicament
A technology of radiopharmaceuticals and auxiliary devices, applied in the directions of radioactive carriers, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of excluding or not including semi-preparative HPLC purification and formulation, and residues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Preparation of radiopharmaceutical (2R, 3R, 11bR)-9-(3-[ 18 F]fluoropropyl)-3-isobutyl-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]-isoquinoline- 2-ol ([ 18 F] FP-(+)-DTBZ).
[0073] Items and materials:
[0074] The aqueous solution containing radionuclide F-18 was produced by Siemens RDS111 cyclotron; the phase transfer catalyst amino polyether 222 and 250mL bagged water for injection were purchased from ABX Chemical Co., Ltd.; K 2 CO 3 , Acetonitrile, and DMSO were purchased from Sigma-Aldrich; ethanol and sodium ascorbate were purchased from Spectrum Chemical Co., Ltd.; QMA, Oasis solid-phase extraction cartridges were purchased from Waters; sterile filter membranes were purchased from Millipore.
[0075] Precursor compound reference [Kung Mei-Ping, Hou Catherine, Goswami Rajesh, PondeDatta E., Kilbourn Michael R., Kung, Hank F..Characterization of optically resolved 9-fluoropropyl-dihydrotetrabenazine as a potential PET imaging agenttargeting ...
Embodiment 2
[0088] Example 2: Preparation of radiopharmaceutical (s)-(-)-N-(1-allylpyrrolidine-2-aminomethyl)-5- fluorine[ 18 F] propyl-2,3-dimethoxybenzamide ([ 18 F]Fallypride)
[0089] Items and materials:
[0090] Precursor compounds were purchased from ABX Chemical Co., Ltd., DMF was purchased from Sigma-Aldrich Company, and the sources of other items were the same as in Example 1.
[0091] Preparation:
[0092] (1) Device installation:
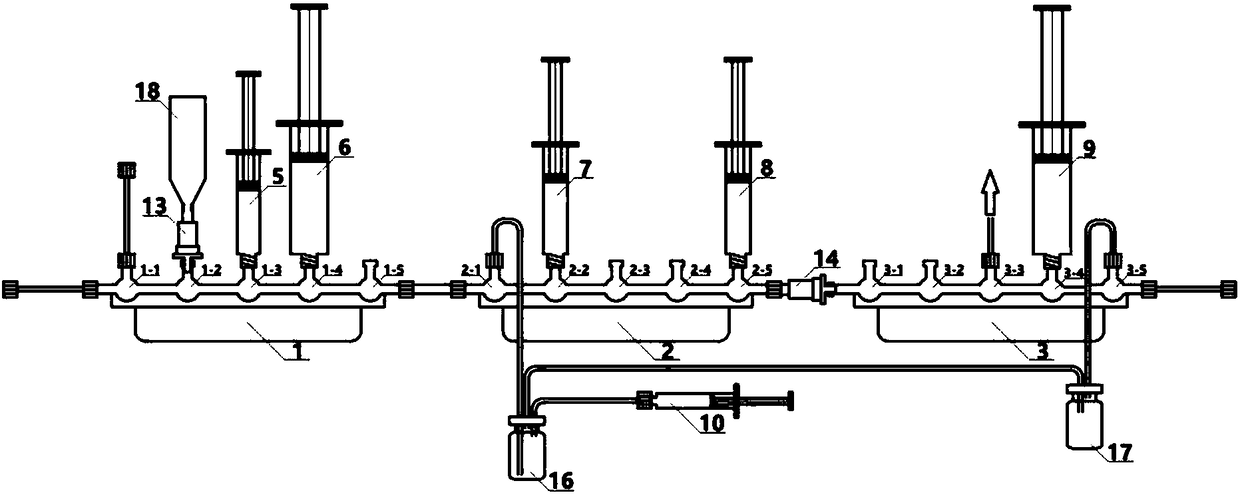
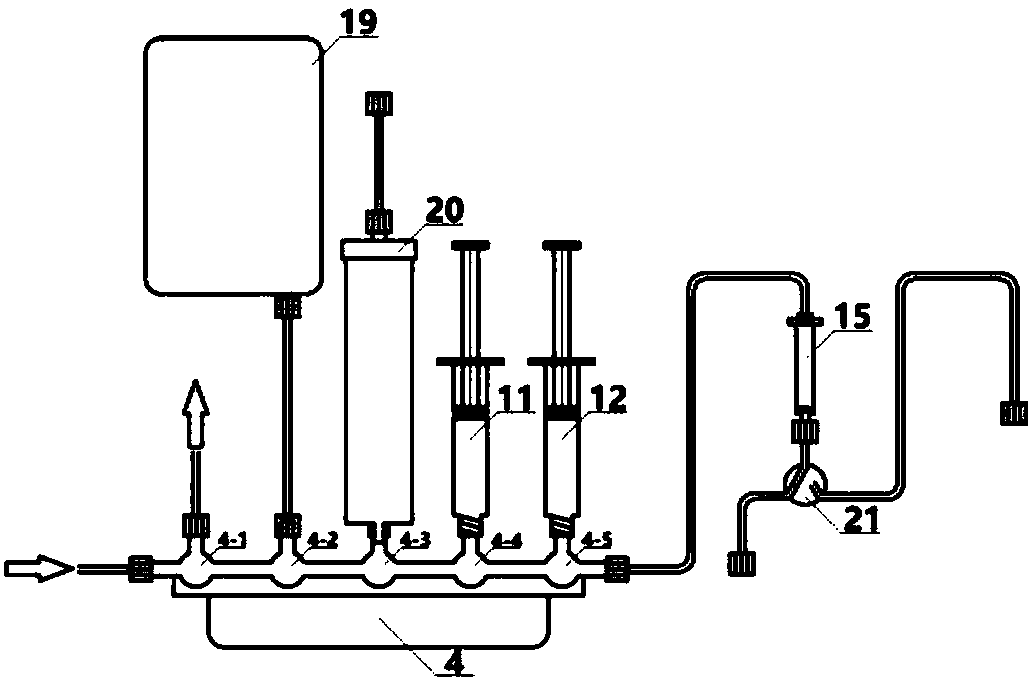
[0093] Install the disposable auxiliary device (disposable ferrule device) on the Neptis Perform synthesis instrument of ORA Company in Belgium (or the TRACERlab MX of GE Company in the United States or the Explora one radiopharmaceutical synthesis instrument of SIEMENS Company in Germany) to ensure that the first reaction is performed five times three times. The through valve 1 to the formulation five-way three-way valve 4 are installed in the corresponding card slots, and the joints are tightly connected, and the first reaction bottle 16 is p...
Embodiment 3
[0102] Example 3: Preparation of radiopharmaceutical (s)-(-)-N-(1-allylpyrrolidine-2-aminomethyl)-5-fluoro[ 18F] propyl-2,3-dimethoxybenzamide ([ 18 F]Fallypride)
[0103] Items and materials:
[0104] Consistent with the source of embodiment 2.
[0105] Preparation:
[0106] (1) Device installation:
[0107] Install the disposable auxiliary device (disposable ferrule device) on the Neptis Perform synthesis instrument of ORA Company in Belgium (or the TRACERlab MX of GE Company in the United States or the Explora one radiopharmaceutical synthesis instrument of SIEMENS Company in Germany) to ensure that the first reaction is performed five times three times. The through valve 1 to the formulation five-way three-way valve 4 are installed in the corresponding card slots, and the joints are fastened. The first reaction bottle 16 is placed in the heating hole of the Neptis Perform type synthesis instrument; the third reaction five-way three-way The thirteenth three-way valve 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com