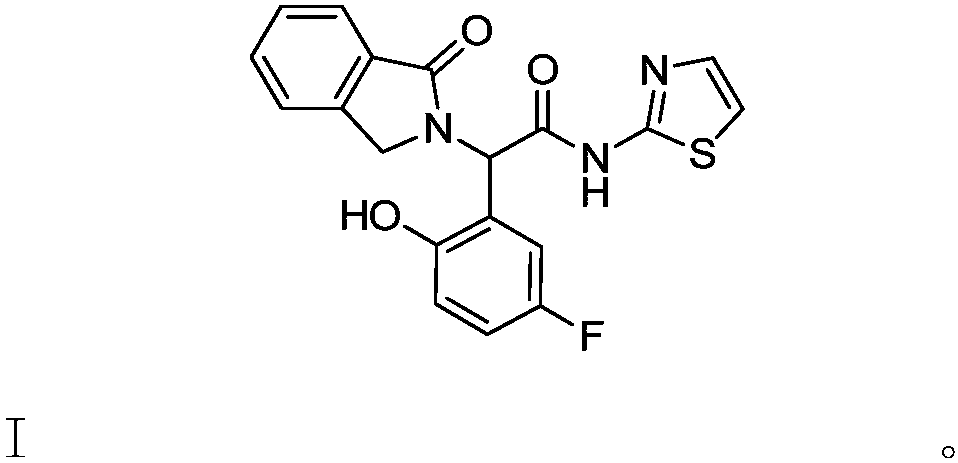
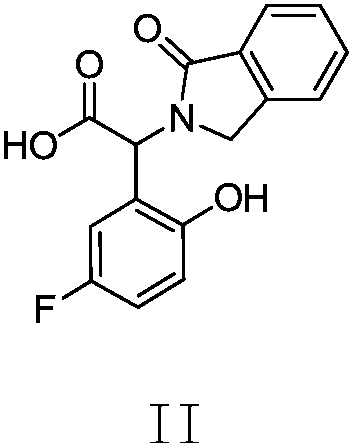
Preparation method, related intermediate, and application of anti-epidermal growth factor receptor drug-resistance mutation inhibitor
A technology for epidermal growth factor and drug resistance, which is applied in the preparation of novel ectopic inhibitors and the preparation of novel inhibitors against epidermal growth factor receptor drug resistance mutations, and can solve the problem of preparing compounds of formula I that have not been reported in published literature. methods, etc., to achieve the effect of good repeatability, easy purification, and process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of 2-(5-fluoro-2-hydroxyphenyl)-2-(1-oxa-2,3-dihydro-1H-2-indenyl)acetic acid II
[0026] Add isoindolin-1-one (13g, 1.0eq (equivalent)) into a 250ml three-necked flask, add trifluoroacetic acid (65g) and concentrated sulfuric acid (26g) under stirring, adjust and control the temperature to maintain at 30°C, Glyoxylic acid monohydrate (9 g, 1.0 eq) was added into the reaction system, and the reaction was stirred at 30° C. for 20 hours. After the reaction was detected by TLC, p-fluorophenol (13.1 g, 1.2 eq) was added to the reaction system, and stirred at 30° C. for 50 hours. After the completion of the reaction detected by TLC, the reaction solution was diluted with water (200 g) and added to 20% sodium hydroxide solution (58.5 g), and the temperature of the mixture was controlled at 20° C. The mixture was stirred for 0.5 hours. After the aqueous phase was extracted twice with isopropyl acetate (500 g each), the organic phases were combined. The organic p...
Embodiment 2
[0029] Example 2 Preparation of 2-(carbonylmethylene)-1-oxa-2-isoindolinium (intermediate III)
[0030] Add isoindolin-1-one (6.5g, 1.0eq) into a 250ml three-necked flask, add acetic acid (123g) and concentrated sulfuric acid (13g) while stirring, adjust and control the temperature to keep at 30°C, add Glyoxylic acid monohydrate (9g, 1.0eq), stirred and reacted at 20-30°C for 20 hours. TLC detected that the reaction was completed to obtain 2-(carbonylmethylene)-1-oxa-2-isoindolinium (intermediate III).
Embodiment 3
[0031] Example 3 Preparation of 2-(5-fluoro-2-hydroxyphenyl)-2-(1-oxa-2,3-dihydro-1H-2-indenyl)acetic acid II
[0032] Add isoindolin-1-one (13g, 1.0eq) into a 50ml three-necked flask, add acetic acid (123g) and concentrated sulfuric acid (26g) under stirring, adjust and control the temperature to keep at 20°C, add ethyl alcohol to the reaction system Aldehydic acid monohydrate (9g, 1.0eq), stirred and reacted at 20°C for 16 hours. After the reaction was detected by TLC, p-fluorophenol (13.1 g, 1.2 eq) was added to the reaction system and stirred at 20-30° C. for 45 hours. After the reaction was detected by TLC, the reaction solution was diluted with water (200 g) and added to 20% sodium hydroxide solution (58.5 g), and the temperature of the mixture was controlled at 10°C. The mixture was stirred for 0.5 hours. After the aqueous phase was extracted twice with isopropyl acetate (500 g each), the organic phases were combined. The organic phase was dried over anhydrous sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com