Compound, nano super-molecular drug carrier and drug containing nano super-molecular drug carrier
A compound and supramolecular technology, applied in the direction of drug combination, preparation for in vivo test, medical preparation of non-active ingredients, etc., can solve the problems of unpredictable photodynamic properties of compounds, complex molecular design manpower and material resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
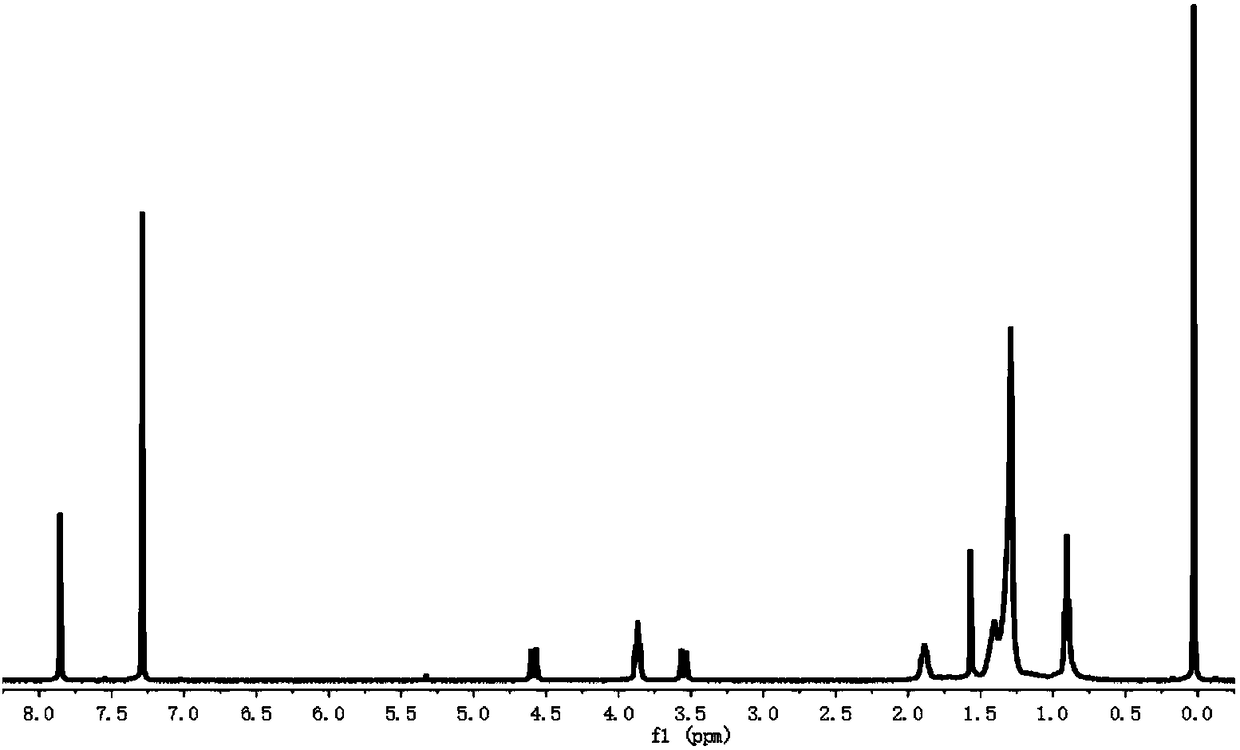
[0058] Synthesis process of 1,5,11,17,23,29-pentanitro-31,32,33,34,35-penta(n-dodecyloxy)calix[5]arene:
[0059] 3.97g of 5,11,17,23,29-penta-tert-butyl-31,32,33,34,35-penta(n-dodecyloxy)calix[5]arene (see Arnaud-Neu , F., Fuangswasdi, S., Notti, A., Pappalardo, S. & Parisi, Angew.Chem., Int.Ed.1998, 37, 112-114 and Garozzo, D., et al.Angew.Chem. , Int.Ed.2005, 44, 4892-4896) was dissolved in 119mL of dry dichloromethane and 34.28mL of acetic acid solution, then gradually added 10.28mL of nitric acid dropwise, and stirred at room temperature for 1 to 4 hours. The color of the solution changed from dark purple to orange. Then add 250mL water to the reaction solution and stir for 30 minutes, extract and separate the mixed solution with saturated sodium carbonate solution, saturated saline solution, and water, collect the organic phase, concentrate, dry over anhydrous sodium sulfate, and recrystallize with dichloromethane and methanol , to obtain 1.74 g of 5, 11, 17, 23, 29-pen...
preparation Embodiment 2
[0076] The preparation process of nano supramolecular drug carrier:
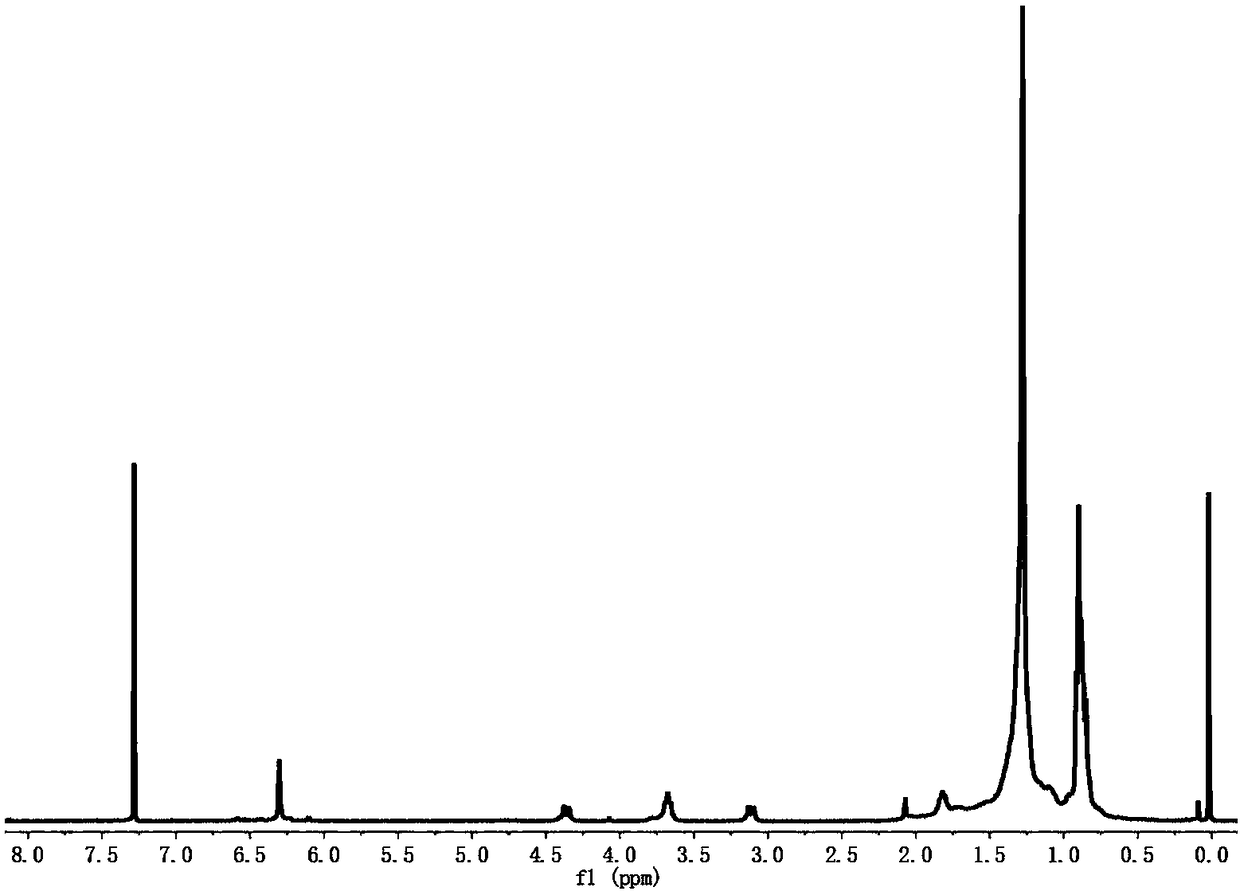
[0077] Mix the methanol solution of GC5A-12C with a concentration of 1mmol / L and the chloroform solution of modified polyethylene glycol with a concentration of 1mmol / L in a ratio of 1:1, then remove the solvent in a vacuum, add water, and then heat and sonicate , and finally obtain the nano-supramolecular drug carrier of GC5A-12C with a concentration of 1 mmol / L. The hydrated particle size of the supramolecular nano drug carrier measured by dynamic light scattering is 113nm, and the polydispersity coefficient is 0.17 ( Figure 7 ); the preparation of the TEM sample, the dispersed nano-carrier solution is taken appropriate amount and dripped on the copper grid supported by carbon, dried at room temperature, then vacuum-dried for 24h, and photographed by TEM ( Figure 8 ); the preparation of the SEM sample, the dispersed nano-carrier solution is taken in an appropriate amount and dropped on a silicon wafer, ...
preparation Embodiment 3
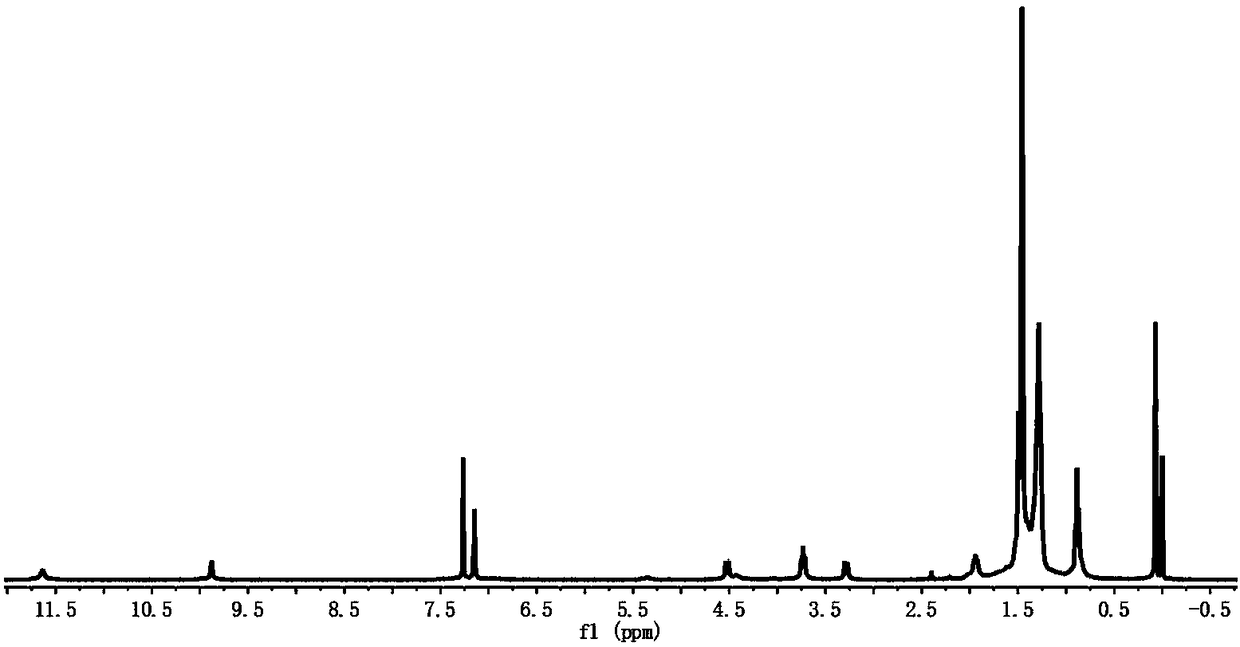
[0079] The preparation process of the drug:
[0080] Weigh 2.383g of N-(2-hydroxyethyl)piperazine-N'-2-ethanesulfonic acid (hereinafter referred to as HEPES), and use ultrapure water to prepare 1L of 10mmol / L pH 7.4 buffer solution (hereinafter referred to as HEPES buffer solution). Weigh A1PcS 4 , with 10mmol / L HEPES buffer solution to prepare 1mmol / L AlPcS 4 stock solution, AlPcS was mixed with 10mmol / L pH 7.4 HEPES buffer solution at 25℃ 4 The stock solution is diluted, and the nano-supramolecular drug carrier obtained in Preparation Example 2, AlPcS 4 The final concentration of the nano-supramolecular drug carrier and the nano-supramolecular drug carrier is 2 μmol / L, and the drug with a concentration of 2 μmol / L is obtained after mixing evenly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com