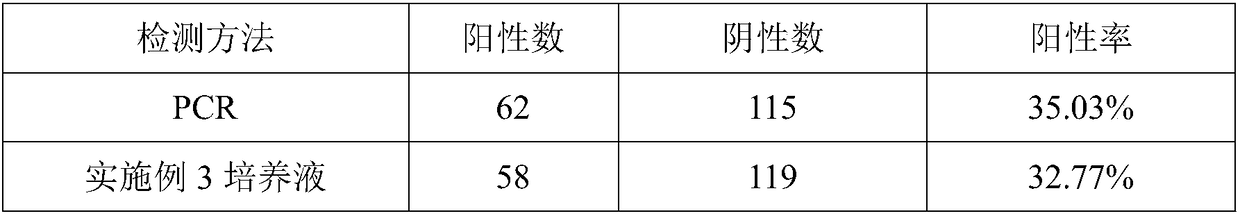
Rapid detection kit for drug susceptibility of mycoplasma bovis and preparation method of rapid detection kit
A detection kit, a technology for Mycoplasma bovis, applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection, etc., can solve the problems of no national standards or patents, and no commercialized kits, etc. Shorten color development time, high efficiency, and reduce false negatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Second aspect, the present invention provides a kind of preparation method of described Mycoplasma bovis drug susceptibility rapid detection kit, comprises the following steps:
[0030] (1) Preparation of Mycoplasma bovis culture medium: dissolve 12g of PPLO powder, 3g of short peptide, 300mg of morroniside, 4g of yeast powder, 2.5g of glucose, and 3.5g of sodium pyruvate in 750mL of ddH2O, add 100mL of MEM medium, 1% (w / v) phenol red solution 2mL, after sterilizing at 116°C for 20min, add 150mL of horse serum inactivated at 56°C and 300,000 units of penicillin, adjust the pH value to 7.6-7.8, dispense into 15mL culture tubes, 9ml / Tube.
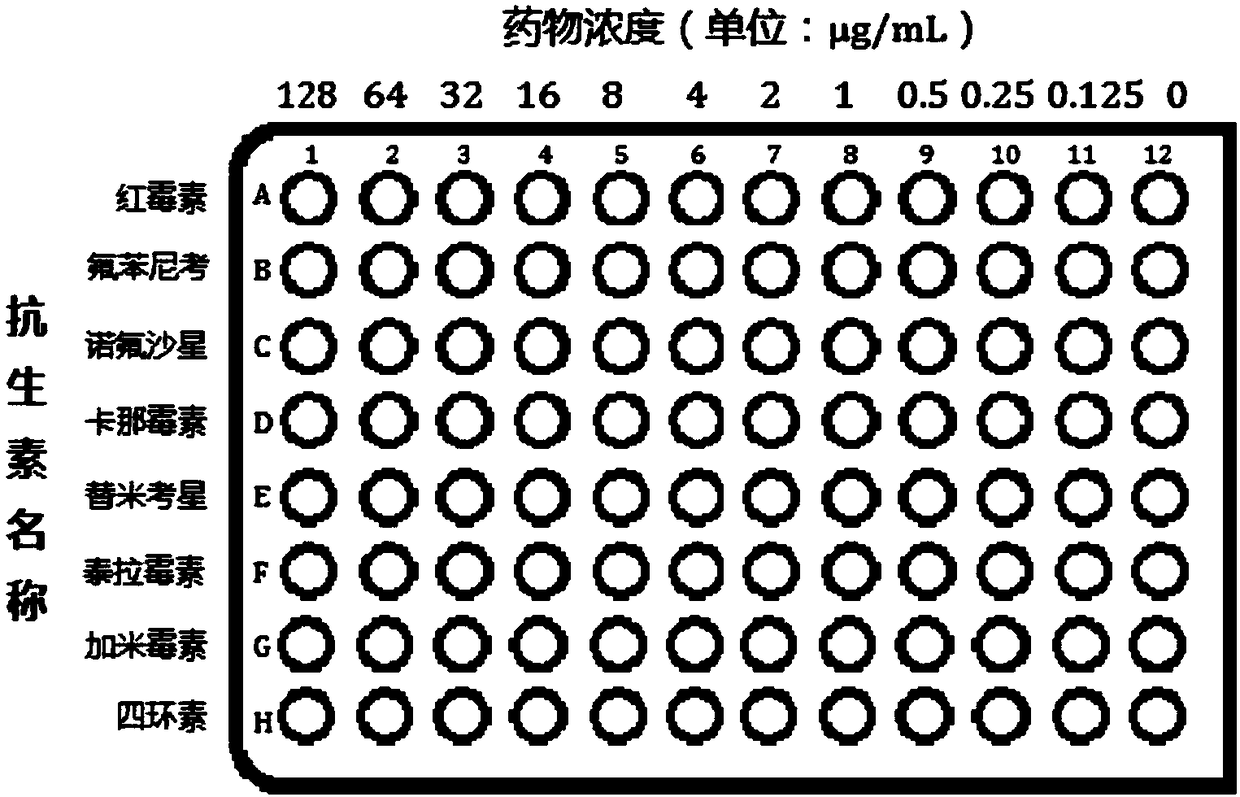
[0031] (2) Preparation of drug sensitive plate:
[0032] S1. The 96-well plate is roughly washed, finely washed, and dried at a constant temperature of 40°C for later use;
[0033] S2. Dissolve erythromycin, florfenicol, norfloxacin, kanamycin, tilmicosin, telamycin, garamimycin and tetracycline in 1-5mL buffer, and dilute to 128μg...
Embodiment 1
[0036] Embodiment 1 a kind of mycoplasma bovis culture fluid
[0037] The Mycoplasma bovis culture solution is composed of the following components and contents: PPLO powder 10-15g, short peptide 2-4g, morroniside 100-500mg, yeast powder 2-6g, glucose 1-3g, sodium pyruvate 1.5-4.5g , MEM medium 80-120 mL, 1% (w / v) phenol red solution 2 mL, horse serum 100-300 mL, 300,000 units of penicillin and ddH 2 O 700-800mL.
[0038] The peptide chain is an oligomeric short peptide of Tyr-Leu-Tyr-Glu-Val-Ala.
Embodiment 2
[0039] Embodiment 2 a kind of mycoplasma bovis nutrient solution
[0040] The Mycoplasma bovis culture solution is composed of the following components and contents: PPLO powder 10-15g, short peptide 2-4g, morroniside 100-500mg, yeast powder 2-6g, glucose 1-3g, sodium pyruvate 1.5-4.5g , MEM medium 80-120 mL, 1% (w / v) phenol red solution 2 mL, horse serum 100-300 mL, 300,000 units of penicillin and ddH 2 O 700-800mL.
[0041] The peptide chain is an oligomeric short peptide of Tyr-Leu-Tyr-Glu-Val-Ala.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com