A kind of preparation method of high mannose type oligosaccharide linked by phytanol
A high mannose and mannose-based technology, applied in the fields of molecular biology and biochemistry, can solve the problems of difficult expression and purification, and difficult to obtain, and achieve the effect of overcoming high efficiency and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Prokaryotic expression and purification of Saccharomyces cerevisiae Alg1DTM, Trx-Alg2, Alg11DTM and Dpm1
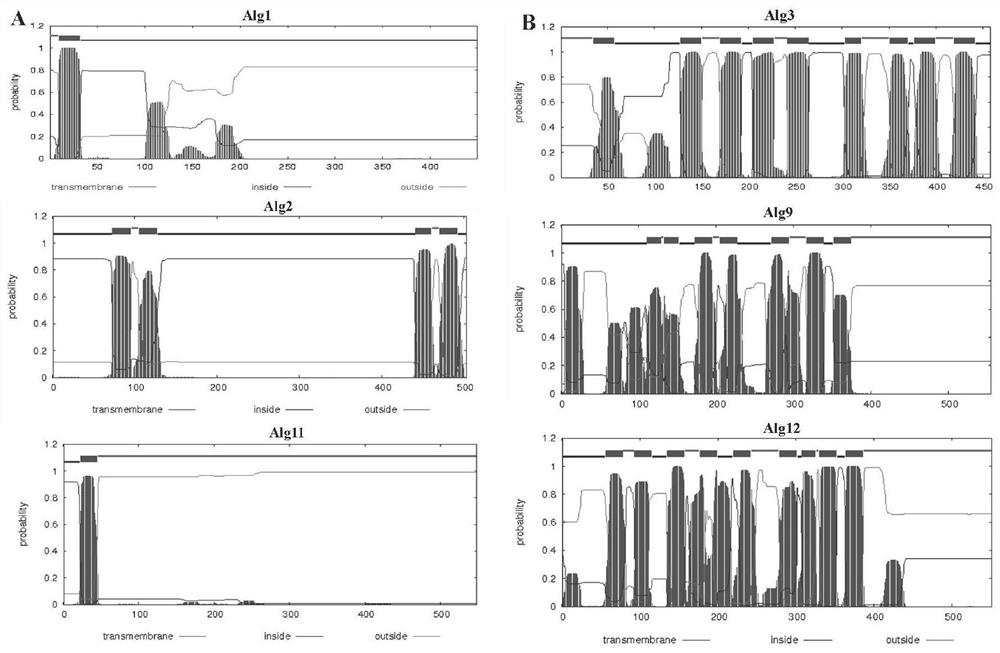
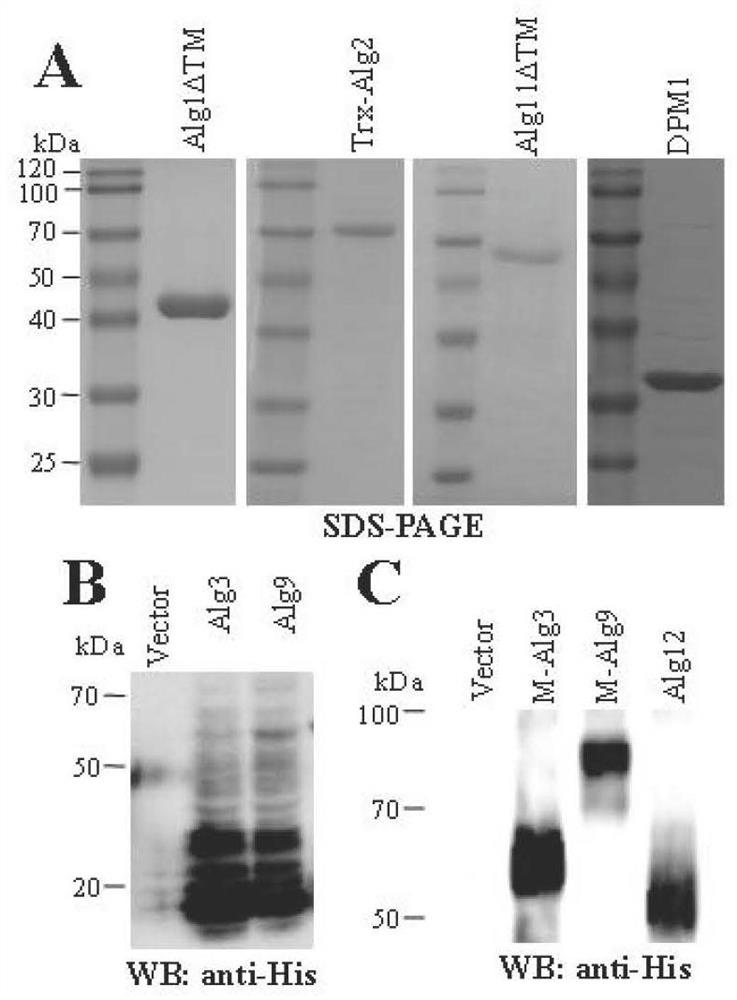
[0029] We used software to analyze the membrane topology of three eukaryotic transmembrane proteins, Alg1, Alg2, and Alg11 (TMHMM Server v. 2.0) ( figure 1A). Alg1 and Alg11 truncated the N-terminal transmembrane structure (Alg1DTM, aa 35-349; Alg11DTM, aa 45-548); Alg2 fused the Trx tag (Trx-Alg2) to construct the following prokaryotic expression vectors: pET28a-Alg1DTM, pET32a- Trx-Alg2 and pET28a-Alg11DTM, and construct vector pET28a-Dpm1. Transform the recombinant prokaryotic expression plasmid into ROSETTA prokaryotic expression host bacteria, spread on LB+ (Kan or Amp) + chloramphenicol (34μg / mL) and pick a single colony from the transformation plate the next day, inoculate in 5mL LB+ (Kan or Amp) + In chloramphenicol liquid medium, culture overnight at 37°C with shaking. Inoculate 2 mL of the overnight cultured bacterial solution into 200 mL of...
Embodiment 2
[0031] Example 2: Prokaryotic expression of Saccharomyces cerevisiae M-Alg3, M-Alg9 and Alg12 and preparation of membrane components
[0032] The membrane topology of three eukaryotic transmembrane proteins of yeast Alg3, Alg9 and Alg12 was analyzed by software (TMHMMServer v. 2.0) ( figure 1 B) Prokaryotic vectors were constructed: pET28a-Alg3, pET28a-Alg9 and pET28a-Alg12, and it was found that both Alg3 and Alg9 were degraded by western blotting ( figure 2 B) Mistic was fused at the N-terminus of Alg3 and Alg9 to construct prokaryotic expression vectors: pET28a-M-Alg3 and pET28a-M-Alg9. First, the corresponding genes were amplified from the Saccharomyces cerevisiae W303A genome and connected to prokaryotic expression Among the vectors, construct recombinant vectors: pET28a-M-Alg3, pET28a-M-Alg9 and pET28a-Alg12. The recombinant prokaryotic expression plasmid was transformed into the ROSETTA prokaryotic expression host strain, and the subsequent induced expression of the p...
Embodiment 3
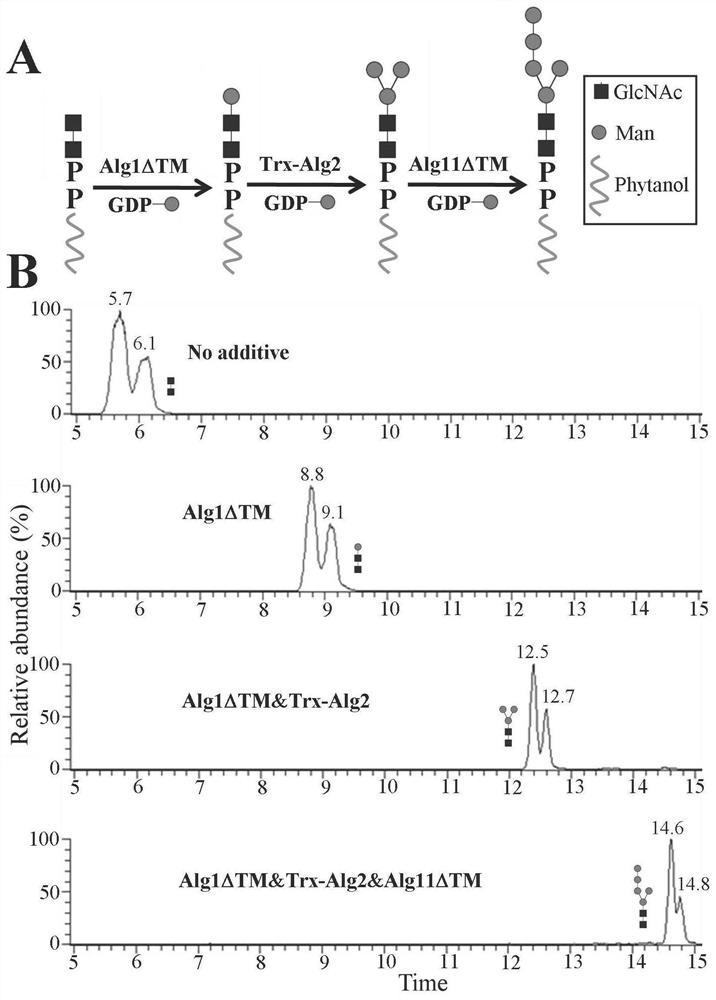
[0033] Example 3: Enzymatic synthesis of phytanyl-phosphate-Man (PP-Man)
[0034] Standard enzyme reaction conditions are as follows (50 µL system): 50 mM Tris / HCl (pH 7.5), 10 mM MgCl 2 , 1% NP-40, 20 mM phytanyl-phosphate, 50 mM GDP-Man and 2 mg / mL purified Dpm1 protein. Reactions were incubated at 30°C for 10 hours. The reaction efficiency of the enzyme was monitored by TLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com