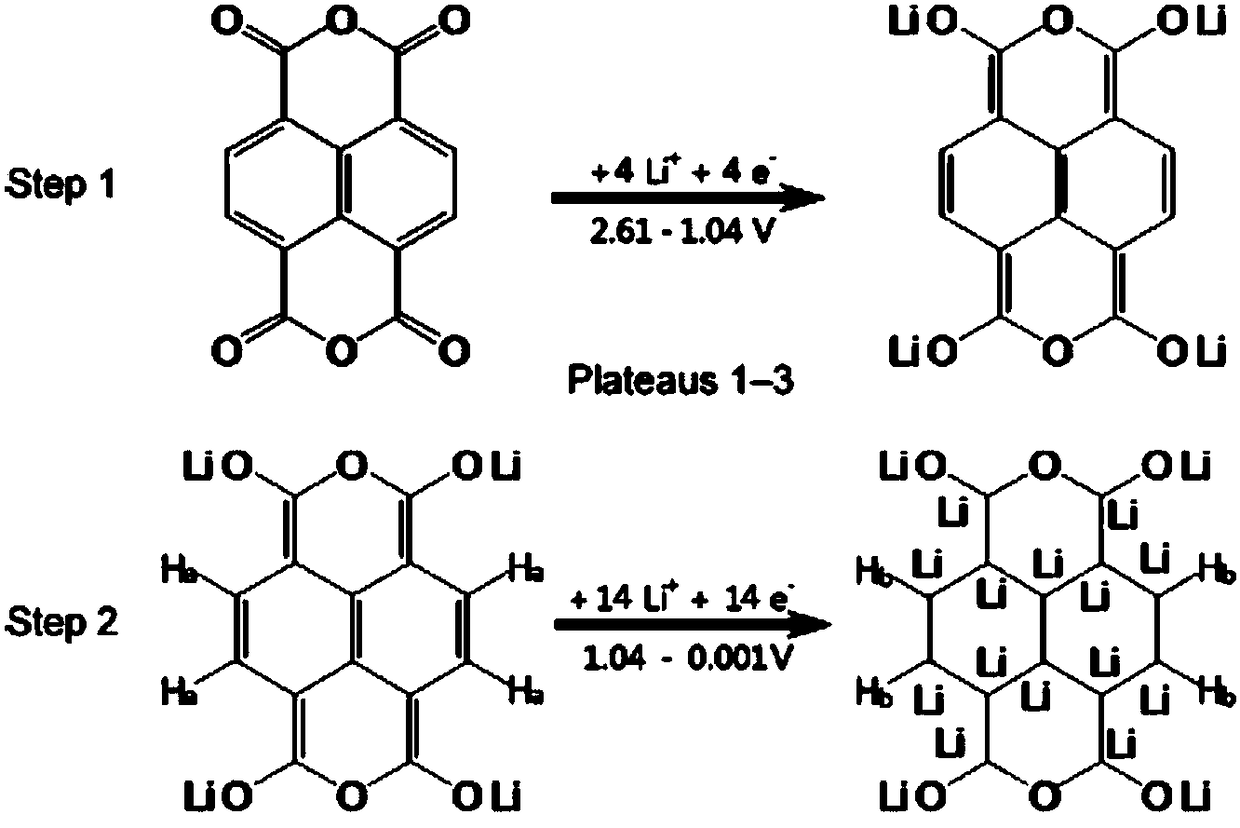
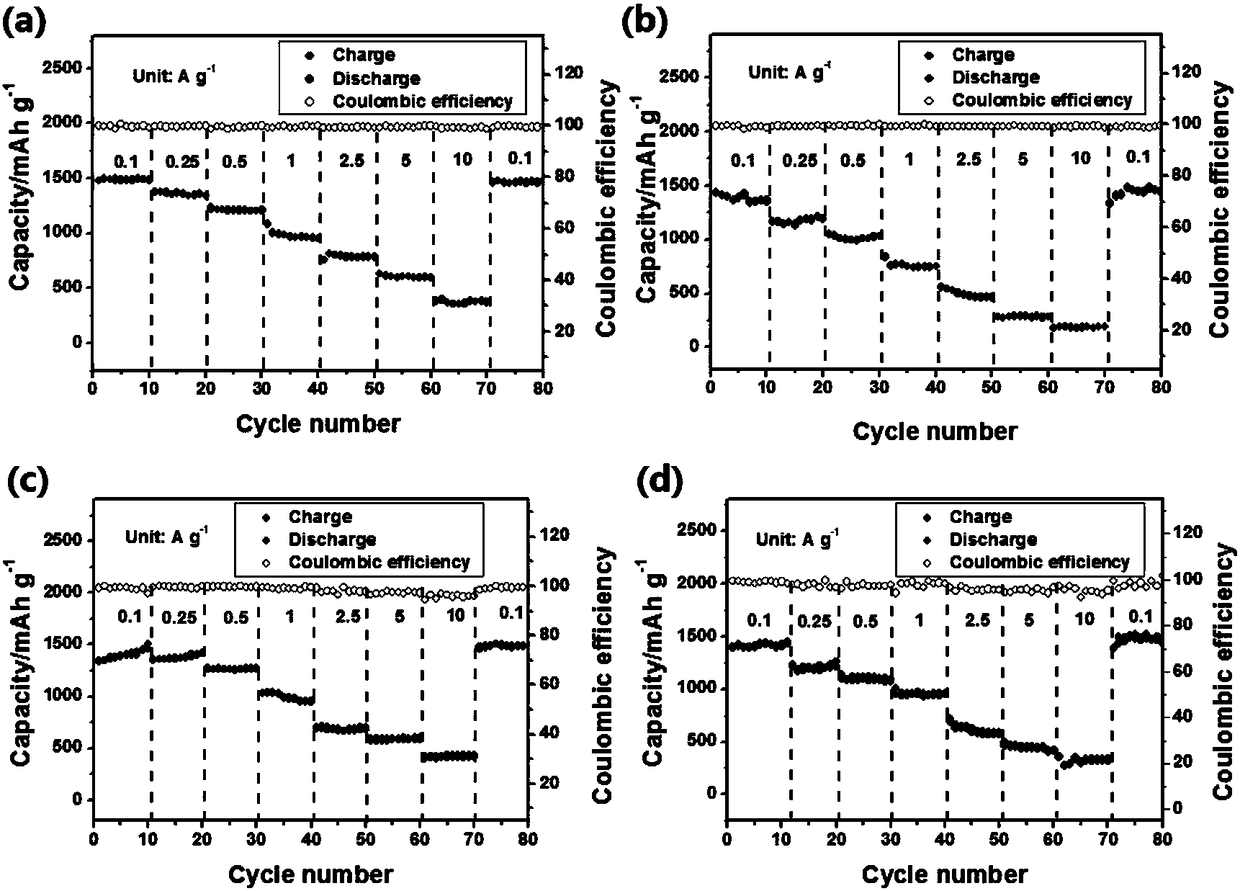
Fused ring compound-based lithium battery negative electrode material and preparation method thereof
A technology of a fused ring pyranyl salt compound and a fused ring pyridine salt compound, which is applied in the field of negative electrode materials for lithium ion batteries, can solve problems such as poor battery cycle stability, achieve strong operability, improve cycle stability, rate performance, and high capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of 4,4-(1,4-phenyl)bis(2,6-diphenylpyranyl boron tetrafluoride) (PBDPT)
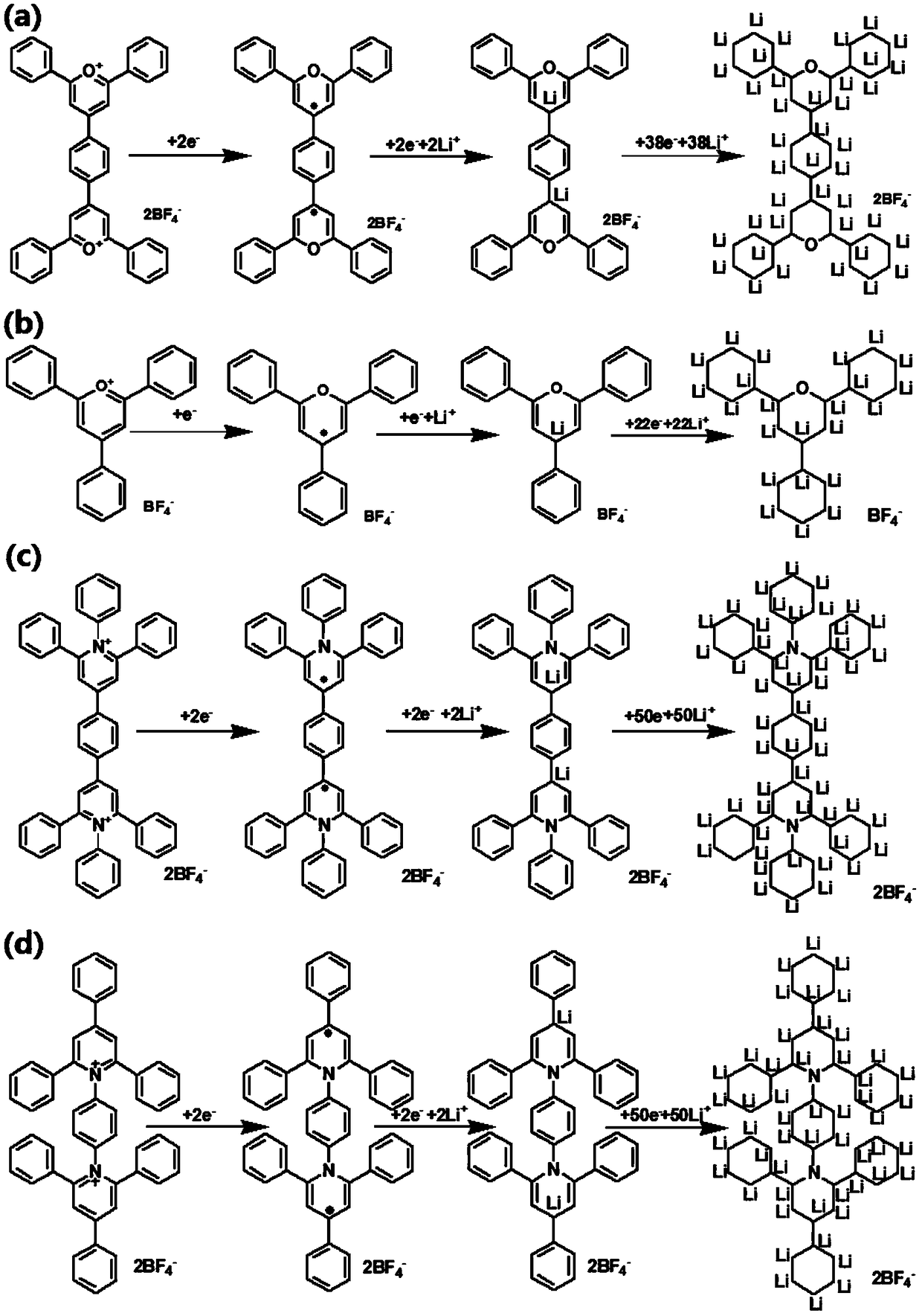
[0039]Terephthalaldehyde (4.948g, 37mmol) and acetophenone (17.7g, 148mmol) were dissolved in 50mL of toluene, then boron trifluoride ether solution (18.75mL) was added, and the resulting mixed solution was refluxed under nitrogen atmosphere for 5 hours, a reaction solution was obtained. The reaction solution was cooled to room temperature, then diethyl ether was added, and the crude product was obtained by filtration. The crude product was dried, recrystallized with acetonitrile, filtered to obtain a yellow solid, and the 4,4-(1,4-phenyl)bis(2, 6-diphenylpyranyl boron tetrafluoride) (PBDPT). The electrochemical mechanism of the deintercalation of lithium ions in PBDPT is as follows: figure 2 (a) shown.
Embodiment 2
[0041] Preparation of 1,4-bis(1,2,6-triphenylpyridine-4-)benzene boron ditetrafluoride (BTPPT)
[0042] Dissolve PBDPT (0.744g, 1.04mmol) and aniline (0.194g, 2.08mmol) in 10mL of dimethyl sulfoxide, then heat the resulting mixed solution to 130°C under a nitrogen atmosphere, and continue the reaction for 24 hours to obtain a reaction solution . The reaction solution was cooled to room temperature, then 300 mL of water was added, and the crude product was obtained by filtration. After the crude product was dried, it was recrystallized with a mixed solvent of methanol / ethanol (v / v, 1 / 1), filtered to obtain a light yellow solid, and the 1 ,4-bis(1,2,6-triphenylpyridine-4-)benzene boron tetrafluoride salt (BTPPT). The electrochemical mechanism of lithium ion deintercalation in BTPPT is as follows: figure 2 (c) shown.
Embodiment 3
[0044] Preparation of 1,1'-p-phenylene-bis(2,4,6-triphenylpyridine) boron tetrafluoride (PPBTPT)
[0045] TPT (0.82g, 2.08mmol) and p-phenylenediamine (0.11g, 1.04mmol) were dissolved in 10mL of dimethyl sulfoxide, and the resulting mixed solution was heated to 130°C under a nitrogen atmosphere, and the reaction was continued for 24 hours. A reaction solution was obtained. The reaction solution was cooled to room temperature, then 300 mL of water was added, and the crude product was obtained by filtration. After the crude product was dried, it was recrystallized with a mixed solvent of methanol / ethanol (v / v, 1 / 1), filtered to obtain a light yellow solid, and the 1 , 1'-p-phenylene-bis(2,4,6-triphenylpyridine) boron tetrafluoride (PPBTPT). The electrochemical mechanism of lithium ion deintercalation of PPBTPT is as follows: figure 2 (d) shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com