A kind of preparation method of hydrogen fluoride, the preparation method of hydrofluoric acid
A technology of hydrogen fluoride and hydrogen fluoride, which is applied in the field of hydrogen fluoride preparation, can solve the problems of low production efficiency of hydrogen fluoride and incomplete decomposition of hydrogen fluoride, and achieve the effects of reducing incomplete decomposition, reducing energy consumption and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
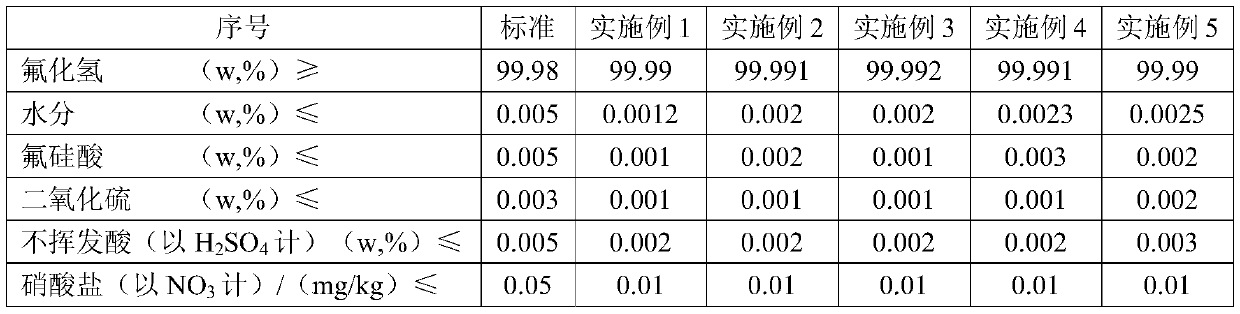
Examples
Embodiment 1
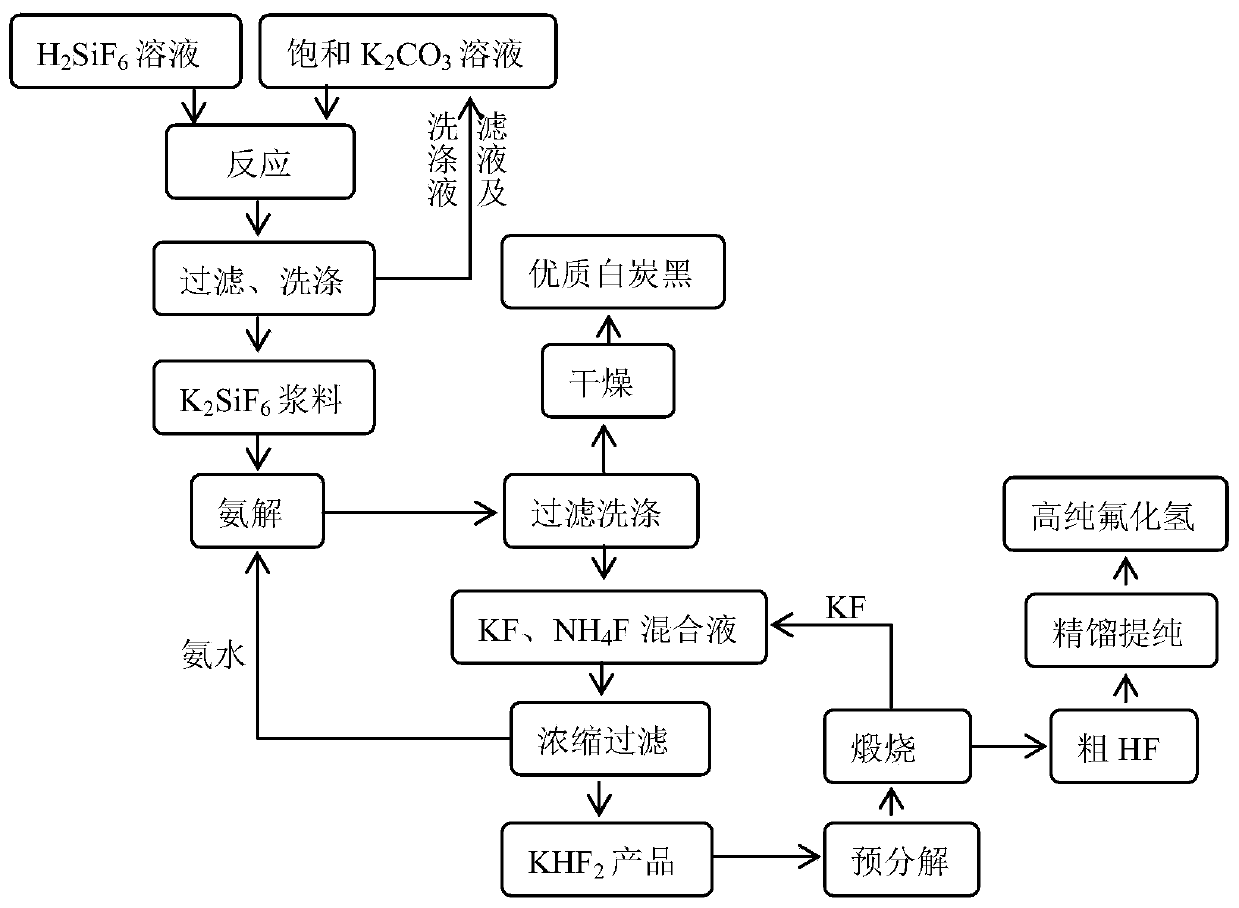
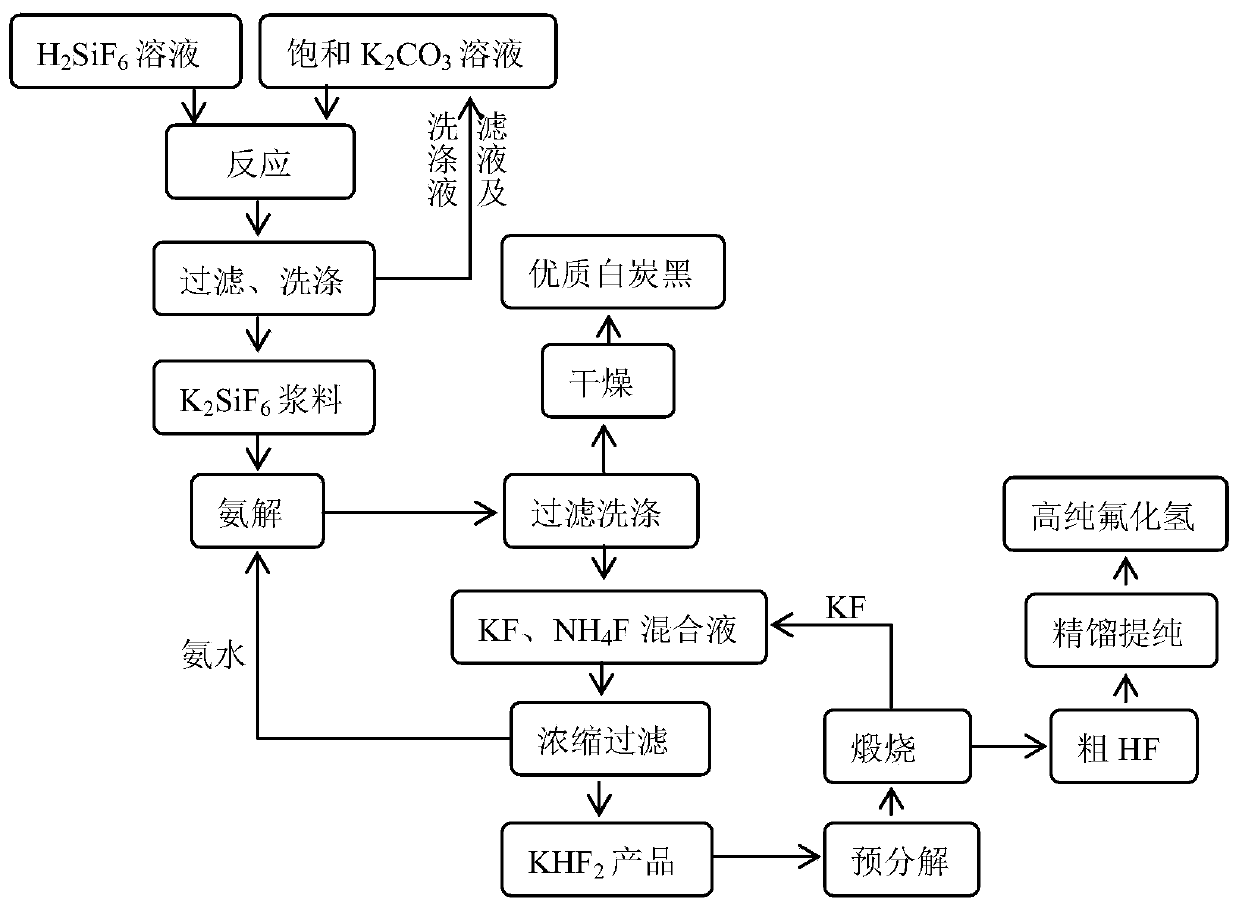
[0038] The preparation method of hydrogen fluoride of the present embodiment, process flow sheet figure 1 , including the following steps:
[0039] 1) Take 1 ton of fluorosilicic acid solution with a mass fraction of 10%, add 0.88m 3 The saturated potassium carbonate solution (20°C, potassium carbonate solubility 111g / L) was reacted at 90°C for 40min to obtain potassium fluorosilicate product slurry, and then filtered for the first time, and the filter cake obtained by the first filtration was washed to obtain Potassium fluorosilicate ointment (contains about 152.86kg potassium fluorosilicate), add water to make 30% (mass fraction) potassium fluorosilicate slurry; the filtrate obtained from the first filtration and the filter cake obtained from the first filtration are washed The final washing solution is used to prepare saturated potassium carbonate solution;
[0040] 2) Warm up the obtained potassium fluorosilicate slurry to 40°C, then pass through 17% ammonia water to car...
Embodiment 2
[0045] The preparation method of the hydrogen fluoride of the present embodiment comprises the following steps:
[0046] 1) Take 1 ton of fluorosilicic acid solution with a mass fraction of 16%, add 1.38m 3 The saturated potassium hydroxide solution was reacted at 90°C for 40 minutes to obtain potassium fluorosilicate product slurry, and then filtered for the first time, and the filter cake obtained by the first filtration was washed to obtain potassium fluorosilicate ointment (containing fluorosilicic acid Potassium is about 244.58kg), add water and be mixed with the potassium fluorosilicate slurry of 30% (mass fraction); The filtrate that filter obtains for the first time and the washing liquid after filter cake washing that filter gained for the first time are used for preparing saturated potassium carbonate solution;
[0047] 2) Warm up the obtained potassium fluorosilicate slurry to 40°C, then pass through 17% ammonia water to carry out ammonolysis for 30 minutes, and th...
Embodiment 3
[0052] The preparation method of the hydrogen fluoride of the present embodiment comprises the following steps:
[0053] 1) Take 1 ton of fluorosilicic acid solution with a mass fraction of 40%, add 3.45m 3 The saturated potassium hydroxide solution was reacted at 90°C for 40 minutes to obtain potassium fluorosilicate product slurry, and then filtered for the first time, and the filter cake obtained by the first filtration was washed to obtain potassium fluorosilicate ointment (containing fluorosilicic acid Potassium is about 611.45kg), add water and be mixed with the potassium fluorosilicate slurry of 30% (mass fraction); The filtrate that filter obtains for the first time and the washing liquid after filter cake washing that filter gained for the first time are used for preparing saturated potassium carbonate solution;
[0054] 2) Warm up the obtained potassium fluorosilicate slurry to 40°C, then pass through 17% ammonia water to carry out ammonolysis for 30 minutes, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com