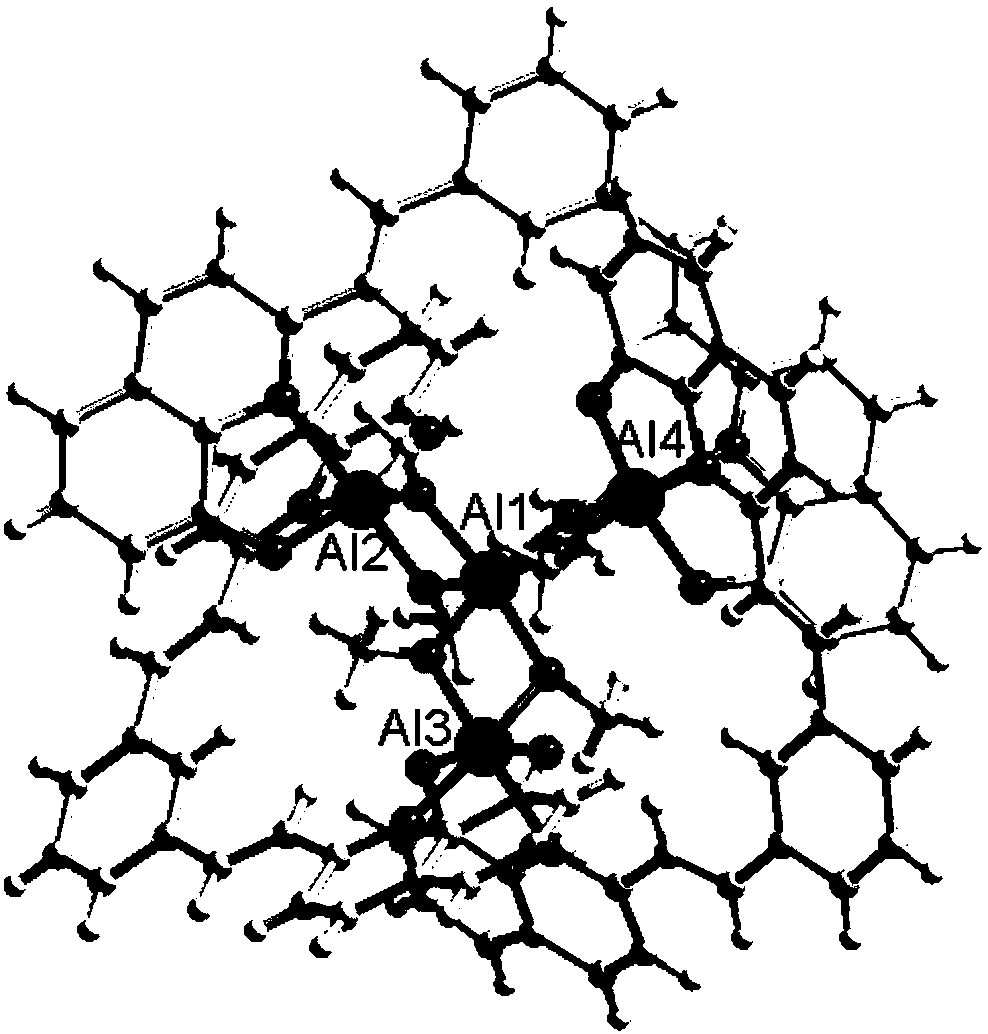
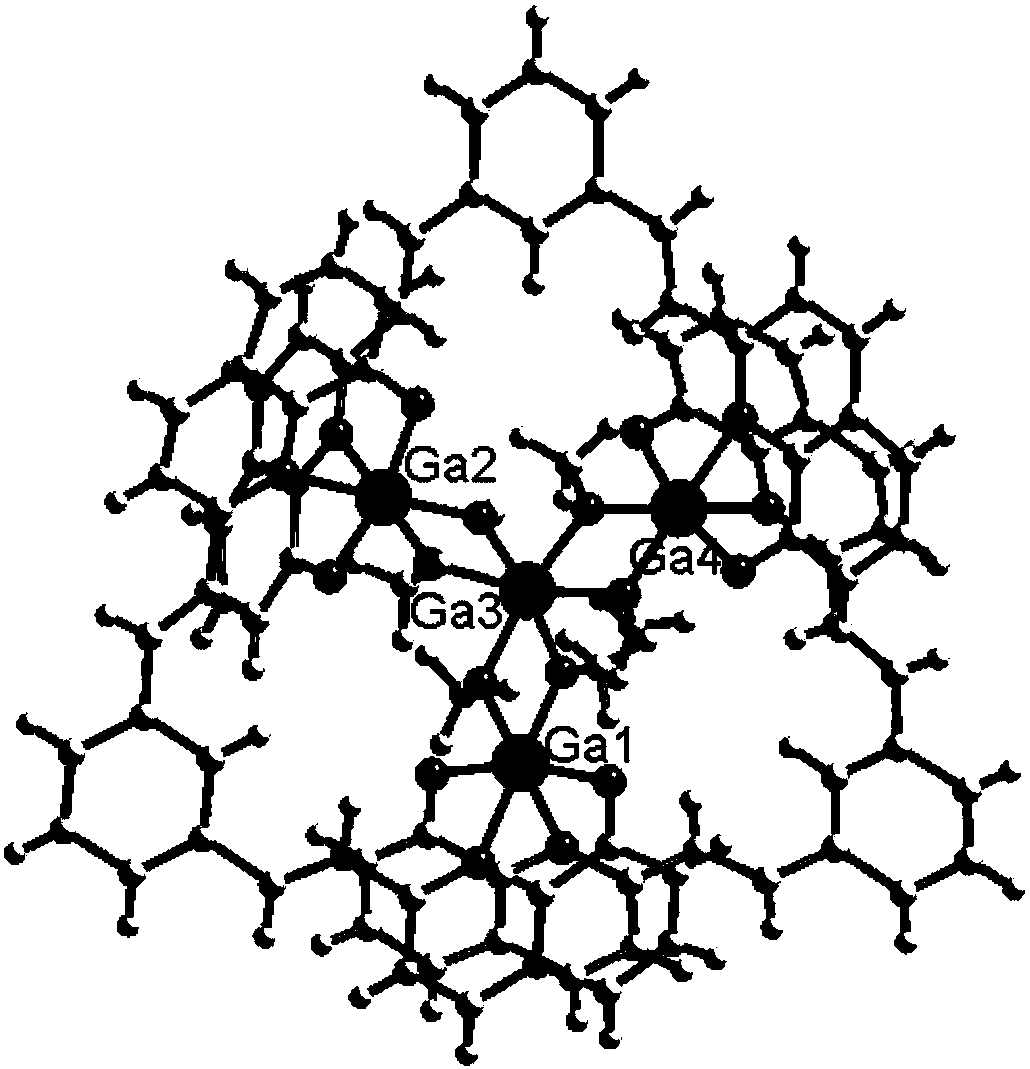
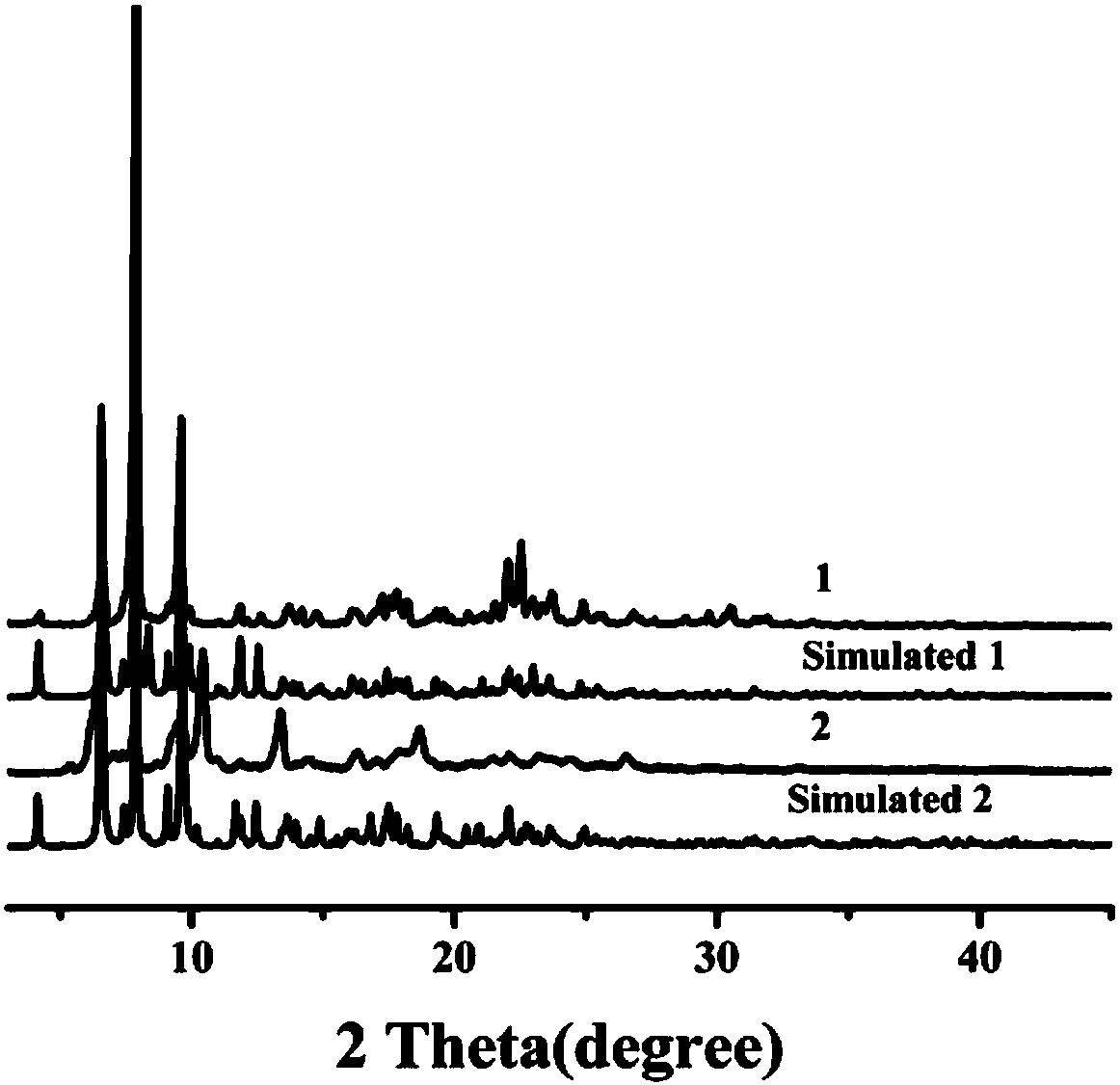
Preparation method and application of 8-hydroxyquinoline complexes with four-core structures
A technology of hydroxyquinoline and complexes, applied in the field of metal-organic coordination chemistry, can solve the problems that the luminous intensity and quantum yield of the device cannot reach the practical stage, hinder the controllable structure and good luminous performance, and low cost, and achieve Broad commercialization prospects, large-scale production, and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also provides a method for preparing the above-mentioned 8-hydroxyquinoline aluminum and gallium complex with a tetranuclear structure, wherein the preparation method comprises: an organic ligand with a structure of formula (II), a metal salt (aluminum salt or gallium salt) aqueous solution and organic solvent are mixed and reacted to prepare two crystalline complexes of structure (I).
[0034]
[0035] In the reaction of preparing the two crystalline complexes of the structure (I), the source of the organic ligand using the structure of the formula (II) is not particularly limited, it can be purchased or made by itself according to the existing literature, and the metal salt is preferably Aluminum nitrate and gallium nitrate, the concentration of the metal salt solution is preferably 0.02 to 0.1mol / L, more preferably 0.08mol / L; the organic solvent is preferably N,N-dimethylformamide and methanol; the The volume ratio of the organic solvent N,N-...
Embodiment 1
[0041] The preparation of formula (II) compound: take by weighing 8-hydroxyquinaldine (10mmol), m-phthalaldehyde (5mmol) in the 25mL double-necked round-bottomed flask of oven dry, add acetic anhydride (10mL) in reaction bottle, N 2 Under protection, it was heated to 120°C for 72h. After cooling to room temperature, a yellow solid was obtained, which was washed three times with distilled water and dried to obtain the target product, yield: 2.05 g, 82%. Dissolve (E,E')-2,2-(1,3-divinylphenyl)bis-8-acetoxyquinaldine (2.0 g, 4 mmol) obtained above in 60 mL of pyridine, Then, 15 mL of distilled water was added to the reaction flask, heated to reflux, and reacted overnight. Cool to room temperature, remove pyridine by distillation under reduced pressure, add 200 mL of distilled water, filter to obtain a yellow solid, wash with distilled water, and dry in vacuo to obtain the target product (E,E')-2,2-(1,3-divinylphenyl) Bis-8-hydroxyquinoline. Yield 1.4g, 84%. 1 HNMR (DMSO-d 6...
Embodiment 2
[0043] Synthesis of 8-hydroxyquinoline aluminum complex (1) shown in formula (I): (E, E')-2,2-(1,3-divinylphenyl) bis-8-hydroxyquinoline (10mmol) was dissolved in N,N-dimethylformamide and methanol mixed solvent (12mL, V / V=1:5), Al(NO 3 ) 3 9H 2 O (20mmol) aqueous solution (0.08mol / L) was added dropwise in the solution, the mixed solution was sealed in a 80mL reactor, kept at 70°C for 20 hours, filtered to obtain yellow blocky crystals, washed with methanol and ether and placed in Air dry. Yield 5.45 g, 85%. ESI-MS m / z: 1537 ([M+1] + );C 88 h 68 o 12 N 6 Al 4 Elemental Analysis Calculated: C70.02, H 4.54, N 5.57; Found: C 69.98, H 4.48, N 5.54. FTIR (KBr tablet): 3443.64(s), 2836.42(w), 1669.54(w), 1599.93(m), 1558.08(s), 1505.81(s), 1455.43(s), 1384.61(s), 1344.48(m ), 1307.51(m), 1279.84(w), 1163.43(w), 1106.94(s), 1059.35(w), 969.55(m), 886.40(w), 832.04(m), 804.50(w), 753.91(s ), 700.06(w), 666.55(w).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com