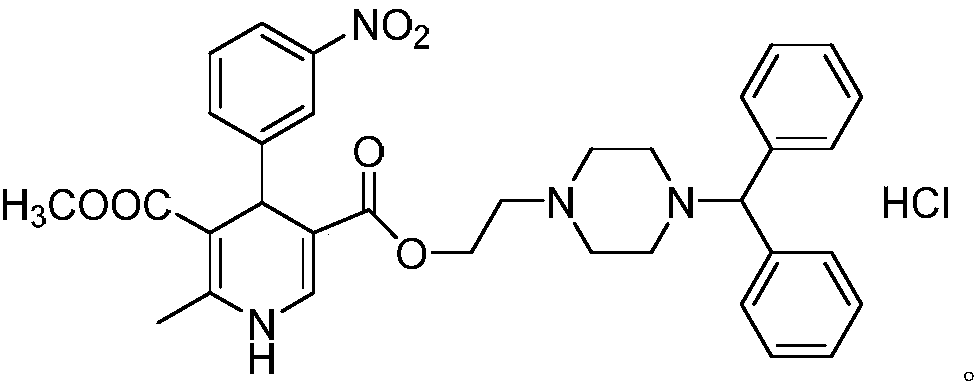
Preparation process of optically-pure manidipine hydrochloride for treating high blood pressure
A technology of manidipine hydrochloride and preparation process, applied in the directions of organic chemistry, organic chemistry methods, etc., can solve the problems of long time, low resolution selectivity, low yield, etc., and achieves high yield and ee value, huge The effect of economic value and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of (S)-manidipine
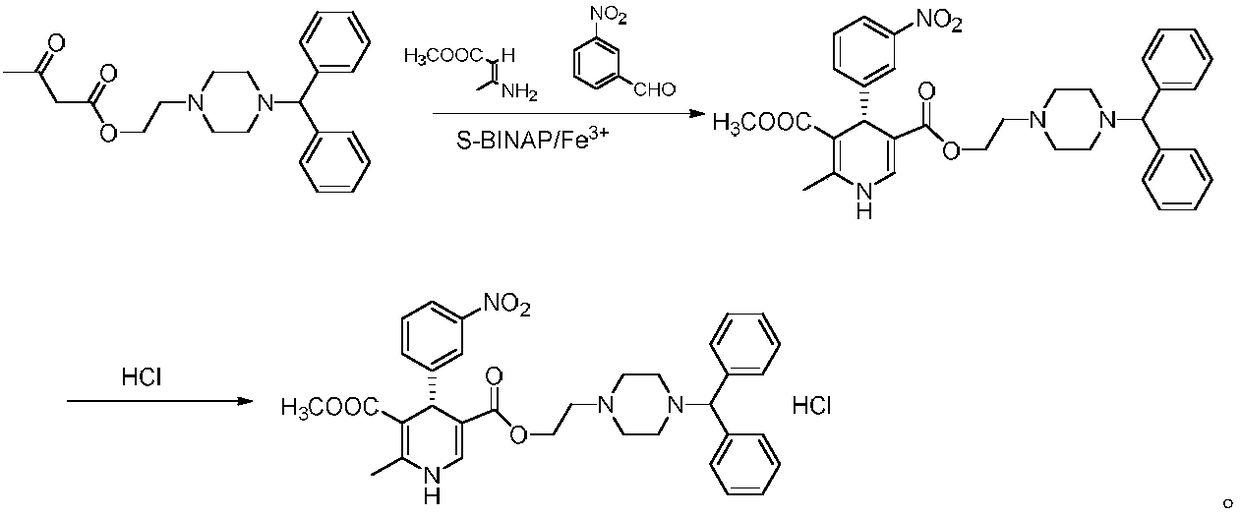
[0030] 4.56 g (12 mmol) of 2-(4-benzyl-1-piperazinyl) ethyl acetoacetate, 1.51 g (10 mmol) of m-nitrobenzaldehyde, and 1.21 g of methyl 3-aminocrotonate were sequentially added. (10.5mmol), (S)-BINAP 0.62g (1mmol), ferric chloride 0.4g (2.5mmol) and 50ml N,N-dimethylformamide were added to the reaction vessel, and the temperature was raised to 100°C for reaction for 2 hours. Concentrated under pressure, recrystallized from n-hexane, and dried to obtain 4.68 g of (S)-manidipine with a yield of 78.4% and an ee value of 99.37%.
Embodiment 2
[0032] Preparation of (S)-manidipine
[0033] 4 g (10.5 mmol) of 2-(4-benzyl-1-piperazinyl) ethyl acetoacetate, 1.51 g (10 mmol) of m-nitrobenzaldehyde, and 1.27 g of methyl 3-aminocrotonate were sequentially added. (11mmol), (S)-BINAP 0.93g (1.5mmol), ferric chloride 0.65g (4mmol) and 50ml of N,N-dimethylformamide were added to the reaction vessel, the temperature was raised to 110°C and the reaction was carried out for 4 hours, and the pressure was reduced. Concentrated, recrystallized from n-hexane, and dried to obtain 4.55 g of (S)-manidipine with a yield of 76.2% and an ee value of 99.33%.
Embodiment 3
[0035] Preparation of (S)-manidipine
[0036] 4.19 g (11 mmol) of 2-(4-benzyl-1-piperazinyl) ethyl acetoacetate, 1.51 g (10 mmol) of m-nitrobenzaldehyde, and 1.38 g of methyl 3-aminocrotonate were sequentially added. (12mmol), (S)-BINAP 0.31g (0.5mmol), ferric bromide 0.59g (2mmol) and 50ml of toluene were added to the reaction vessel, the temperature was raised to 90° C. and reacted for 2 hours, concentrated under reduced pressure, and recrystallized from n-hexane, After drying, 4.66 g of (S)-manidipine was obtained, with a yield of 78.1% and an ee value of 99.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com