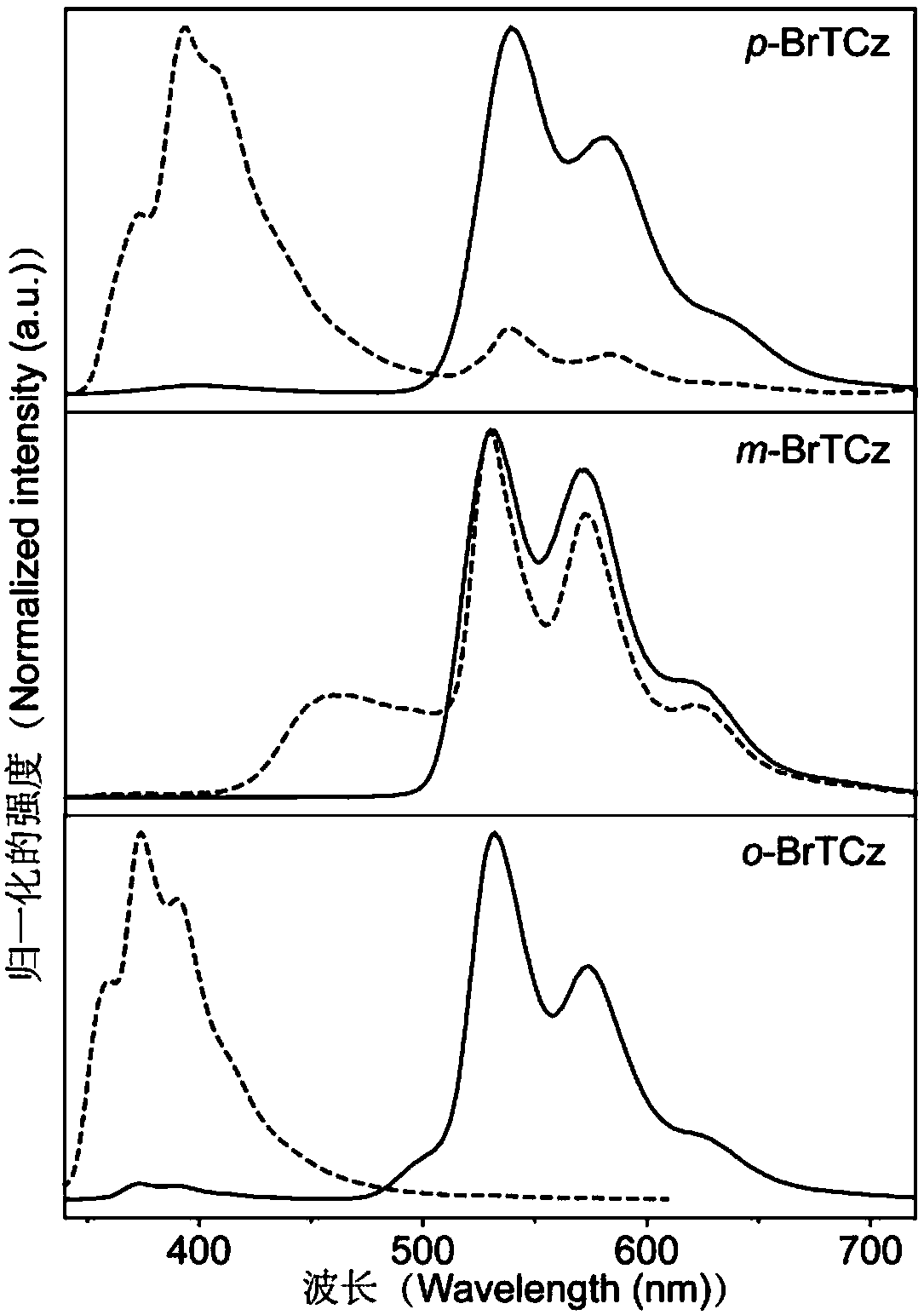
Preparation and mechanism research of efficient phosphorescent pure organic long-afterglow material
A high-efficiency phosphorescence, long-term technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., to achieve the effects of cheap raw materials, high quantum efficiency, and long afterglow life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis process of pure organic long afterglow materials:
[0034] Weigh 5 g (29.9 mmol) of carbazole in a 250 mL flask, fill it with nitrogen three times, add 100 mL of distilled tetrahydrofuran solution, stir in an ice-water bath, slowly add 22.4 mL of n-butyllithium (concentration: 1.6 mol / L) dropwise, Stir in an ice-water bath for one hour. In another 250mL two-necked flask, add 5.5g (29.9mmol) of trichlorotriazine, fill with nitrogen three times, add 50mL of distilled tetrahydrofuran solution, and stir in an ice-water bath. Water bath reaction for four hours. The obtained mixed solution was suction-filtered, washed with acetone, dried, and used as the raw material for the second step reaction. The reaction product is named triazine monocarbazole starting material.
[0035] Weigh 0.823g (4.76mmol) of p-bromophenol and 0.254g (6.344mmol, solid content: 60%) of sodium hydride in a 50mL flask, put it in an ice-water bath, slowly add distilled tetrahydrofuran s...
Embodiment 2
[0037] Take by weighing m-bromophenol 0.823g (4.76mmol), sodium hydride 0.254g (6.344mmol, solid content is 60%) in the flask of 50mL, put into the ice-water bath, slowly drop the distilled tetrahydrofuran solution, stir, have A large number of bubbles are generated, the solution is light brown, and becomes clear after 30 minutes. Weigh 0.5g (1.586mmol) of triazine monocarbazole raw material into a 100mL flask, add 60mL tetrahydrofuran, stir, and add the p-bromophenol mixed solution into triazine monocarbazole The azole was refluxed and stirred for two hours. Spin-dried, petroleum ether: dichloromethane = 4:1, separated by chromatographic column, and synthesized pure organic long-lasting material to obtain a white solid m-BrTCz (0.63g, yield rate 67.0%), the structural characterization is as follows: 1 H NMR(d-DMSO):8.13(d,2H),8.07(d,2H),7.77(t,2H),7.68(d,2H),7.55(t,2H),7.45(tt,2H), 7.36(t,2H),7.19(t,2H); 13 C NMR (CDCl3): δ172.89, 165.73, 152.62, 138.48, 130.98, 129.53, 127...
Embodiment 3
[0039] Weigh 0.823g (4.76mmol) of o-bromophenol, 0.254g (6.344mmol, solid content: 60%) of sodium hydride in a 50mL flask, put it in an ice-water bath, slowly add distilled tetrahydrofuran solution dropwise, stir, and A large number of bubbles are generated, the solution is light brown, and becomes clear after 30 minutes. Weigh 0.5g (1.586mmol) of triazine monocarbazole raw material into a 100mL flask, add 60mL tetrahydrofuran, stir, and add the p-bromophenol mixed solution into triazine monocarbazole The azole was refluxed and stirred for two hours. Spin-dried, petroleum ether: dichloromethane = 4:1, separated by chromatographic column, and synthesized pure organic long-lasting material to obtain a white solid o-BrTCz (0.53g, yield 57.1%), the structural characterization is as follows: 1 H NMR(d-DMSO):8.10(d,2H),7.91(d,2H),7.78(d,2H),7.63(d,4H),7.43~7.47(m,2H),7.32(t,2H ), 7.07(t,2H). 13 C NMR (CDCl 3 ): δ172.57, 165.65, 149.84, 138.54, 133.82, 128.97, 127.64, 126.83, 126....
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com