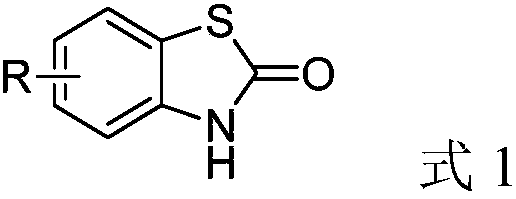
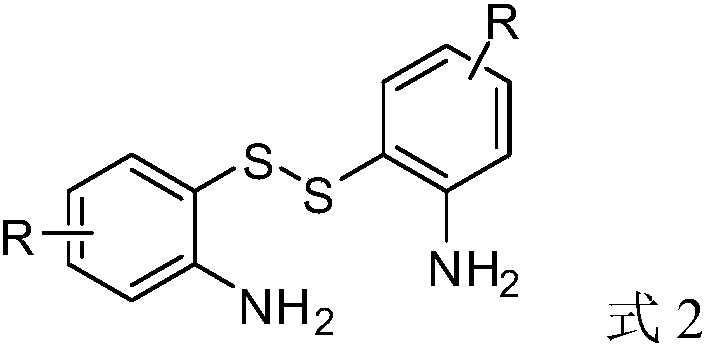
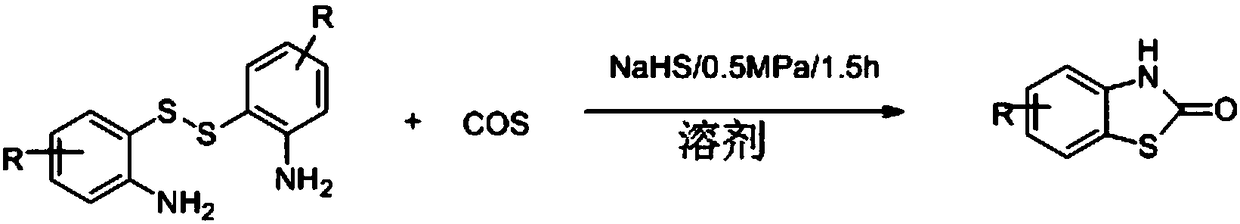
Method for synthesizing benzothiazole-2-ketone derivative from carbonyl sulfide and disulfide as raw materials
A disulfide and benzothiazole technology, which is applied in the field of synthesizing benzothiazol-2-one derivatives, can solve the problem of high cost of raw material preparation of 2-chlorobenzothiazole, long synthesis steps of o-nitrochlorobenzene, O-aminothiophenol instability and other problems, to achieve the effect of improving atom economy, low price, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0033] Synthesis of benzothiazolones from carbonyl sulfide catalyzed by different kinds of inorganic sulfides.
[0034] Put a magnet into a 12mL stainless steel autoclave, and add 0.5mmol o-aminoaromatic disulfide, inorganic sulfide and 1ml solvent in sequence, and tighten the autoclave. Fill the reaction kettle with an appropriate amount of COS, stir for 1.5 hours, stop the reaction, slowly exhaust the gas in the reaction kettle, unscrew the reaction kettle, and transfer the solution in the reaction kettle to a 50mL Erlenmeyer flask. It was extracted with ethyl acetate and washed with saturated brine three times. The organic phases were combined and dried over anhydrous magnesium sulfate for 30 minutes. Anhydrous magnesium sulfate was removed by filtration, and the crude product was obtained by distillation under reduced pressure. Use a mixed solvent of dichloromethane and ethyl acetate as the eluent, dry-pack the column and dry-load the sample, and through column chromatogr...
Embodiment 13-21
[0044] Synthesis of benzothiazolone derivatives.
[0045] Put a magnet into a 12mL stainless steel autoclave, and add 0.5mmol aromatic disulfide, 0.25mmol sodium hydrosulfide and 1ml DMF in sequence, and tighten the autoclave. Fill the reactor with 0.5MPa COS, stir the reaction for 1.5h, stop the reaction, slowly exhaust the gas in the reactor, unscrew the reactor, and transfer the solution in the reactor to a 50mL Erlenmeyer flask. It was extracted with ethyl acetate and washed with saturated brine three times. The organic phases were combined and dried over anhydrous magnesium sulfate for 30 minutes. Anhydrous magnesium sulfate was removed by filtration, and the crude product was obtained by distillation under reduced pressure. Use a mixed solvent of dichloromethane and ethyl acetate as the eluent, dry-pack the column and dry-load the sample, and through column chromatography (200-300 mesh silica gel), obtain solutions containing the two products respectively, and distill u...
Embodiment 13
[0047] (1) Synthesis of 6-methoxybenzothiazol-2-one from 4,4'-dimethoxy-2,2'-dithiodianiline.
[0048] Dry packing Dry loading column chromatography (200-300 mesh silica gel) separation: using gradient elution, ethyl acetate and dichloromethane as eluent, dichloromethane: ethyl acetate (V / V) = 100:1, and then increase the polarity to 20:1, after separation, 163 mg of 6-methoxybenzothiazol-2-one was obtained as a white solid, and the separation yield by column chromatography was 90%.
[0049] (2) Structural identification of 6-methoxybenzothiazol-2-one
[0050]
[0051] Characterization data of 6-methoxybenzothiazol-2-one: 1H NMR (DMSO-d6, 500MHz): δ (ppm) 11.658 (brs, 1H), 7.23 (d, 1H, J=2.5Hz), 7.02 (d, 1H, J = 8.5Hz), 6.86 (dd, 1H, J1 = 8.5Hz, J2 = 2.5Hz), 3.73 (s, 3H); 13C NMR (DMSO-d6, 125MHz): δ (ppm) 169.8 ,155.2,129.9,124.3,113.2,112.1,107.8,55.6; MS(EI):m / z calcd for C 8 h 7 NO 2 S[M] + : 180.9, found 181.0. The melting point is 161-163°C.
[0052] The analys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com