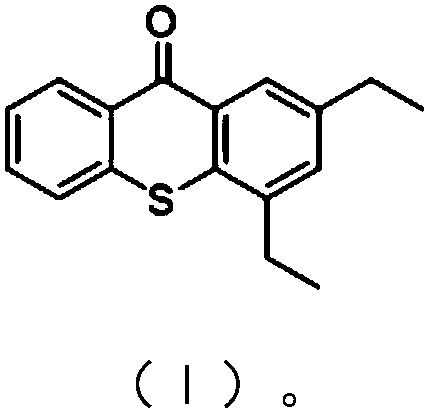
Preparation method of 2,4-diethylthioxanthone
A technology of diethylsulfur and diethylthiophenol, which is applied in the field of compound preparation, can solve the problems of increased waste water discharge, complex preparation process, and difficult storage, and achieves reduced usage, cheap and easy-to-obtain raw materials, and post-production Handle with convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
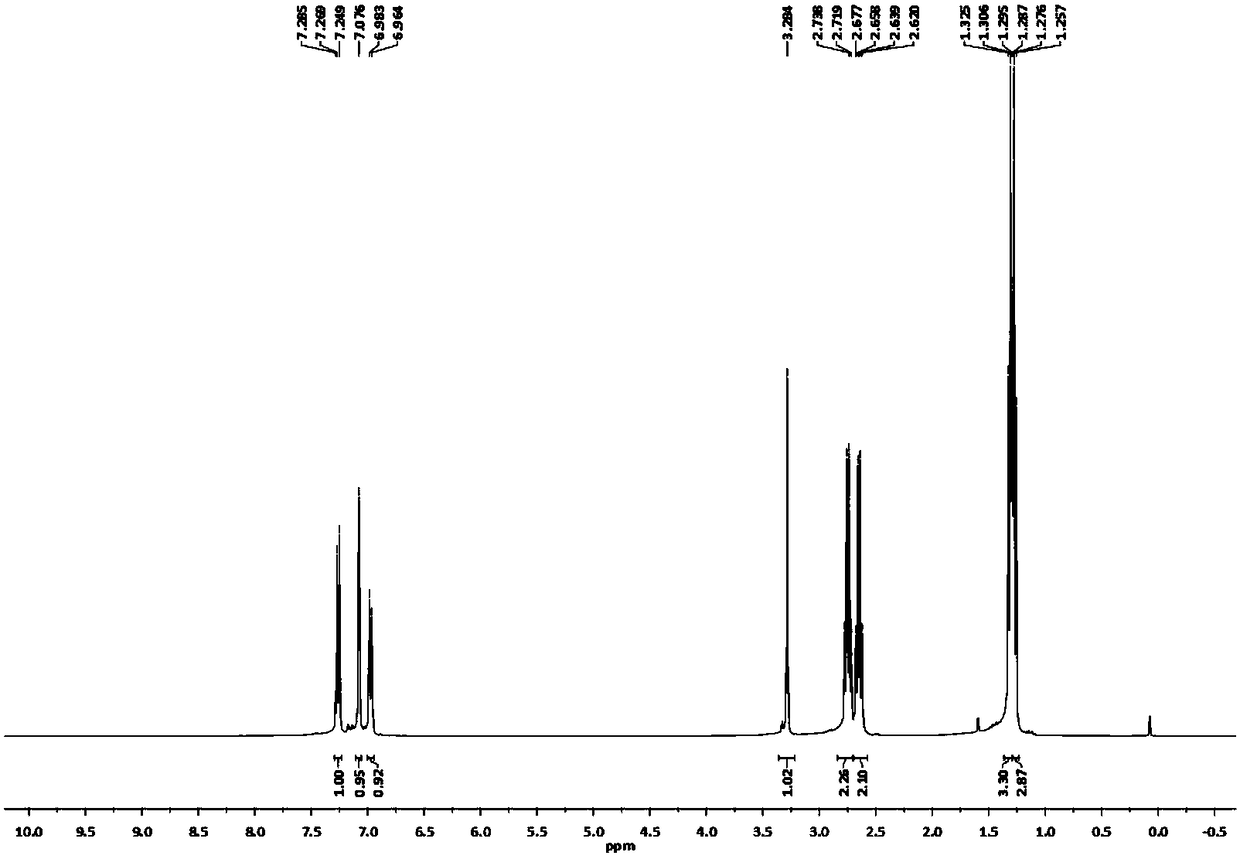
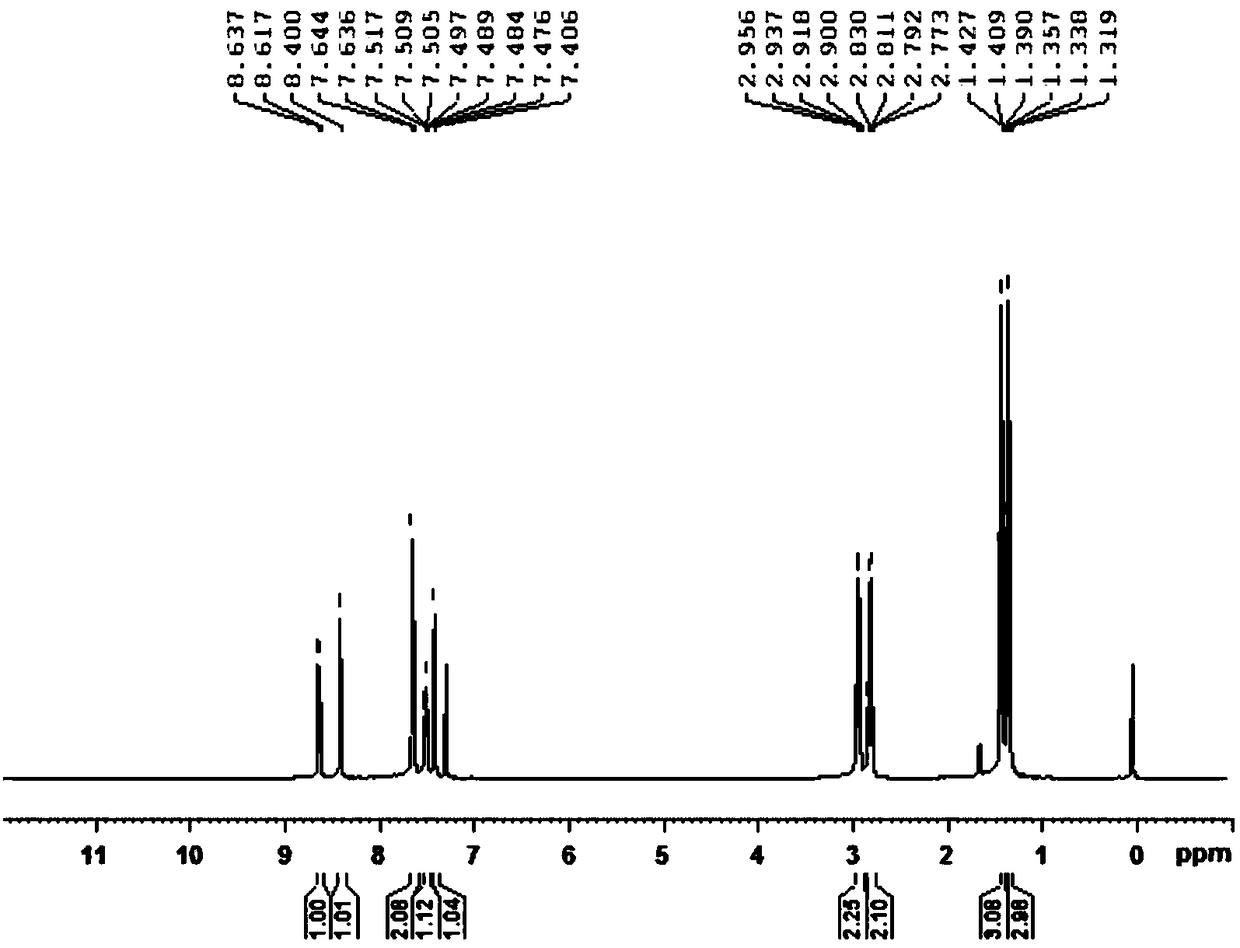
[0030] Add 33.2g (0.20mol) of 2,4-diethylthiophenol, 60mL of N,N-dimethylformamide and 14.16g (0.24mol) of potassium hydroxide into a 500mL four-necked flask, heat to make 2, Completely convert 4-diethylthiophenol into 2,4-diethylthiophenate potassium, then add 33.0g (0.24mol) o-chlorobenzonitrile, heat up to 130°C under stirring, keep warm for reaction, and monitor the reaction by TLC or gas phase After the reaction, the solvent was distilled off under reduced pressure to obtain crude 2-(2,4-diethylthiophenol)benzonitrile. Add the crude product to 70mL of toluene, cool down to below 5°C in an ice bath, slowly add 25mL of 95% sulfuric acid dropwise, after dropping, heat slowly, raise the temperature to 90°C and keep it warm for reaction, monitor the reaction by TLC or gas phase, after the reaction, cool down to room temperature, Add water to the reaction system, stir for 1 hour, let stand, separate the water phase, wash the organic phase with water until neutral, and recover t...
Embodiment 2
[0032] Add 33.2g (0.20mol) of 2,4-diethylthiophenol, 60mL of N,N-diethylformamide and 14.96g (0.22mol) of sodium ethoxide into a 500mL four-necked flask, heat to make 2,4 -Diethylthiophenol is completely converted into 2,4-diethylthiophenate sodium, then add 33.0g (0.24mol) o-chlorobenzonitrile, heat up to 135°C under stirring, keep warm for reaction, TLC or gas phase monitoring reaction, After the reaction, the solvent was distilled off under reduced pressure to obtain crude 2-(2,4-diethylthiophenol)benzonitrile. Add the crude product to 70mL of toluene, cool down to below 5°C in an ice bath, slowly add 30mL of 95% sulfuric acid dropwise, after dropping, heat slowly, raise the temperature to 105°C and keep it warm for reaction, monitor the reaction by TLC or gas phase, after the reaction, cool down to room temperature, Add water to the reaction system, stir for 0.5 hours, let stand, separate the water phase, wash the organic phase with water until neutral, and recover toluene...
Embodiment 3
[0034]Add 33.2g (0.20mol) of 2,4-diethylthiophenol, 30mL of dimethyl sulfoxide, 30mL of m-diethylbenzene and 9.6g (0.24mol) of sodium hydroxide into a 500mL four-neck flask, heat Completely convert 2,4-diethylthiophenol into sodium 2,4-diethylthiophenate, then add 33.0g (0.24mol) o-chlorobenzonitrile, heat up to 135°C under stirring, keep warm for reaction, TLC or The reaction was monitored by gas phase. After the reaction, the solvent was distilled off under reduced pressure to obtain crude 2-(2,4-diethylthiophenol)benzonitrile. Add the crude product to 70mL of toluene, cool down to below 5°C in an ice bath, slowly add 30mL of 95% sulfuric acid dropwise, after dropping, heat slowly, raise the temperature to 100°C and keep it warm for reaction, monitor the reaction by TLC or gas phase, after the reaction, cool down to room temperature, Add water to the reaction system, stir for 0.8 hours, let stand, separate the water phase, wash the organic phase with water until neutral, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com