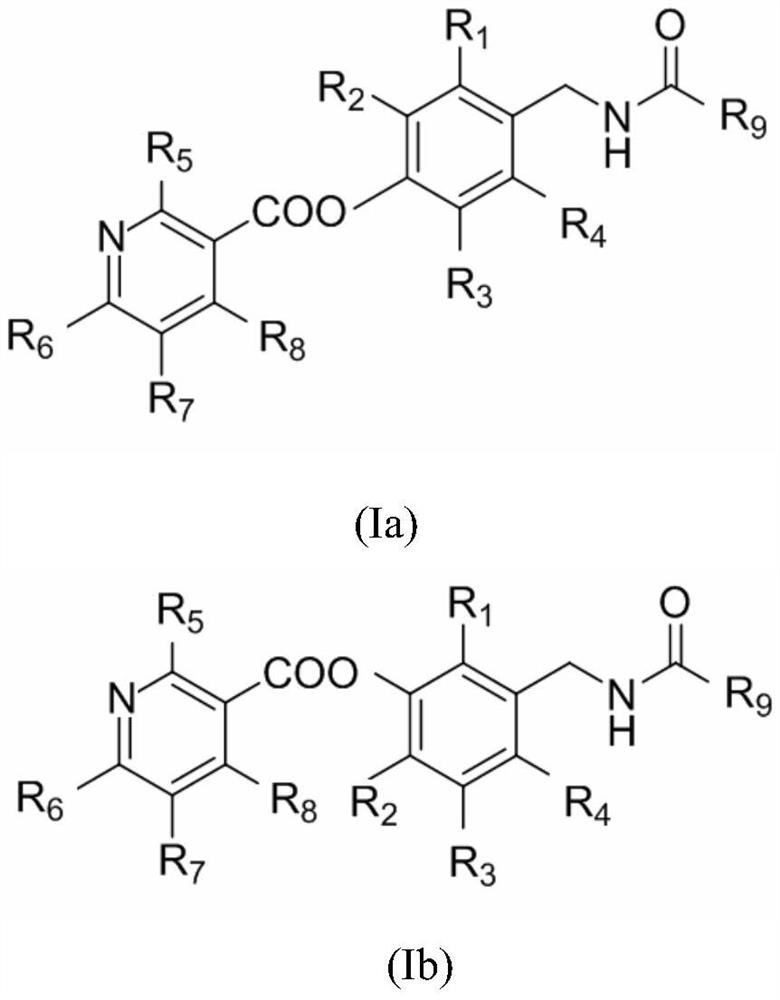
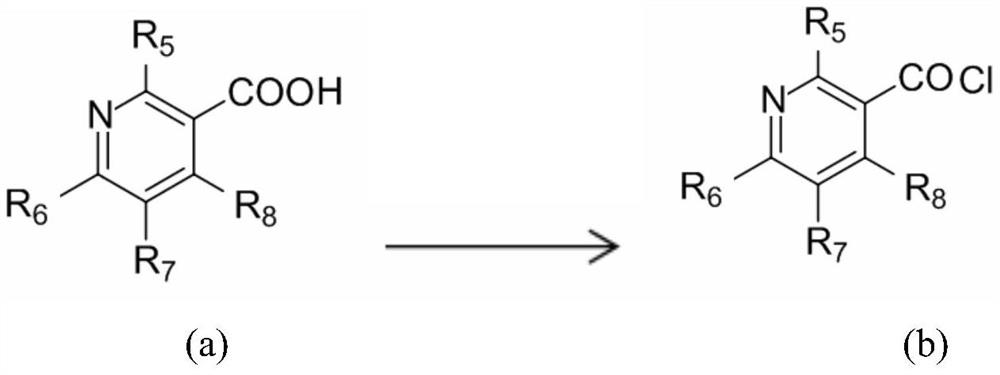
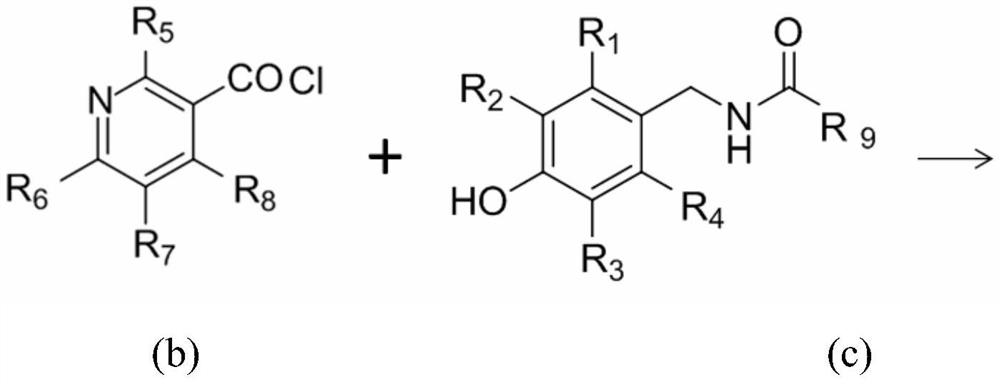
Capsaicin-containing nicotinic acid derivative, preparation method and use thereof
A technology of nicotinic acid esters and compounds, which is applied in the field of capsaicin-containing nicotinic acid capsaicin ester derivatives, preparation and use, can solve the problems of insufficient capsaicin activity, poor water solubility of capsaicin, difficulty in absorbing irritants, etc., and facilitate industrialization Production, appetite-stimulating, and digestion-improving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 8-methyl-N-[(3-hydroxy-4-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide (compound 1, code 1) and 8-methyl- Preparation of N-[(4-hydroxy-3-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide (compound 2, code 2):
[0083]0.1mol 8-methyl-N-[(3,4-dihydroxyphenyl)-methyl]-(trans)-6-nonenylamide was mixed with 0.15mol in 100ml of thionyl chloride at 80°C Nicotinic acid was reacted for 10 hours, poured into an ice-water bath, extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, and then column chromatographed with ethyl acetate:petroleum ether=1:2~1:5 to obtain the target product 8 -Methyl-N-[(3-hydroxy-4-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide, yield 48.8%; and 8-methyl-N-[ (4-Hydroxy-3-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide, yield 44.3%. The relevant data are as follows:
[0084] 8-Methyl-N-[(3-hydroxy-4-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide (Compound 1) MS(EI,70ev)m / z: 370; Anal. Calcd. for C21H26O4N2: C, 68.21, H...
Embodiment 2
[0088] Preparation of 8-methyl-N-[(3-methoxy-4-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide (Compound 3):
[0089] Dissolve 0.2mol of methyl iodide and 0.1mol of 8-methyl-N-[(3-methoxy-4-hydroxyphenyl)-methyl]-(trans)-6-nonenylamide in 300ml of acetone , add 12 grams of catalyst K2CO3, and react with 0.15mol nicotinic acid at 80°C for 3 hours to obtain the target product 8-methyl-N-[(3-methoxy-4-nicotinate phenyl)-methyl]- (trans)-6-Nonenylamide. The relevant data are as follows:
[0090] MS (EI, 70ev) m / z: 384; Anal. Calcd. for C22H28O4N2: C, 68.75, H, 7.29, N 7.29; Found C, 68.70, H, 7.25, N 7.24.
Embodiment 3
[0092] Preparation of 8-methyl-N-[(4-methoxy-3-nicotinate phenyl)-methyl]-(trans)-6-nonenylamide (Compound 4)
[0093] According to the operation of Example 2, just use 8-methyl-N-[(4-methoxy-3-hydroxyphenyl)-methyl]-(trans)-6-nonenylamide instead of 8-methyl -N-[(3-methoxy-4-hydroxyphenyl)-methyl]-(trans)-6-nonenylamide to give the compound 8-methyl-N-[(4-methoxy- 3-Nicotinate phenyl)-methyl]-(trans)-6-nonenylamide. The relevant data are as follows:
[0094] MS (EI, 70ev) m / z: 384; Anal. Calcd. for C22H28O4N2: C, 68.75, H, 7.29, N 7.29; Found C, 68.70, H, 7.25, N 7.24.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com