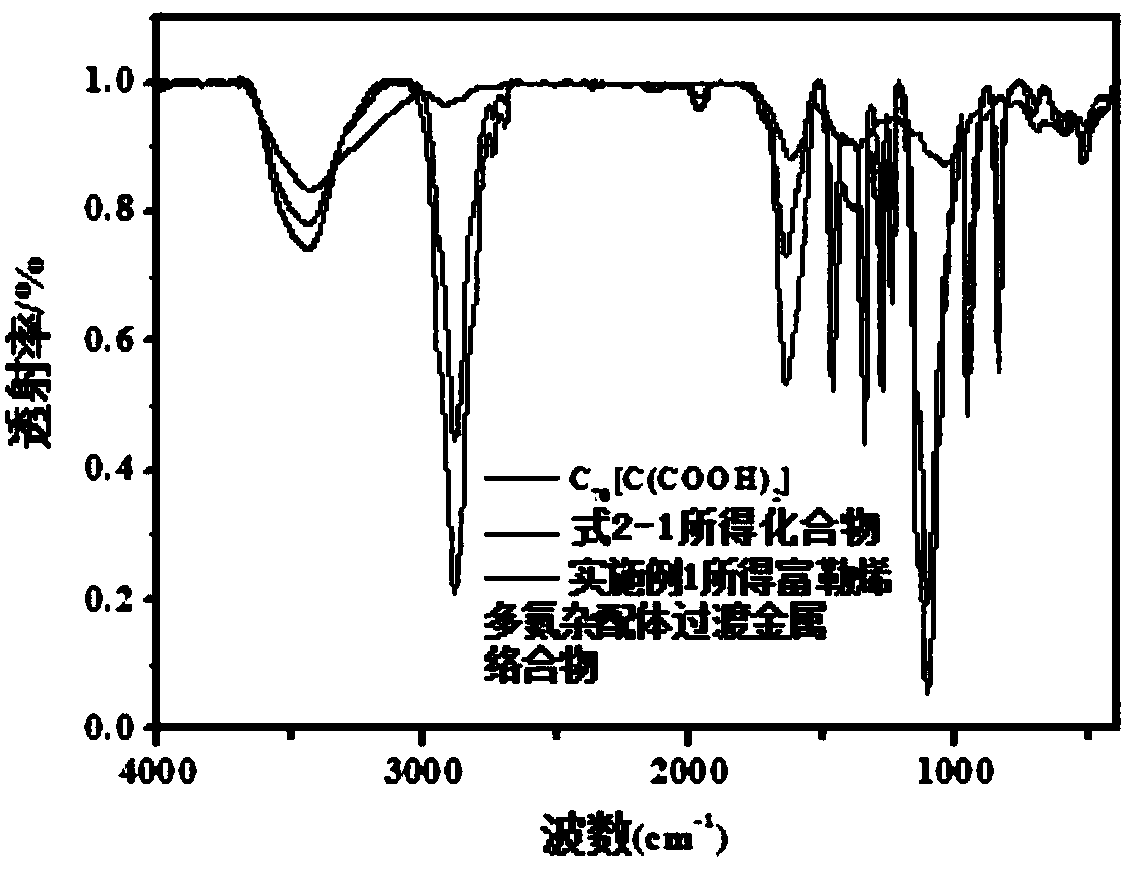
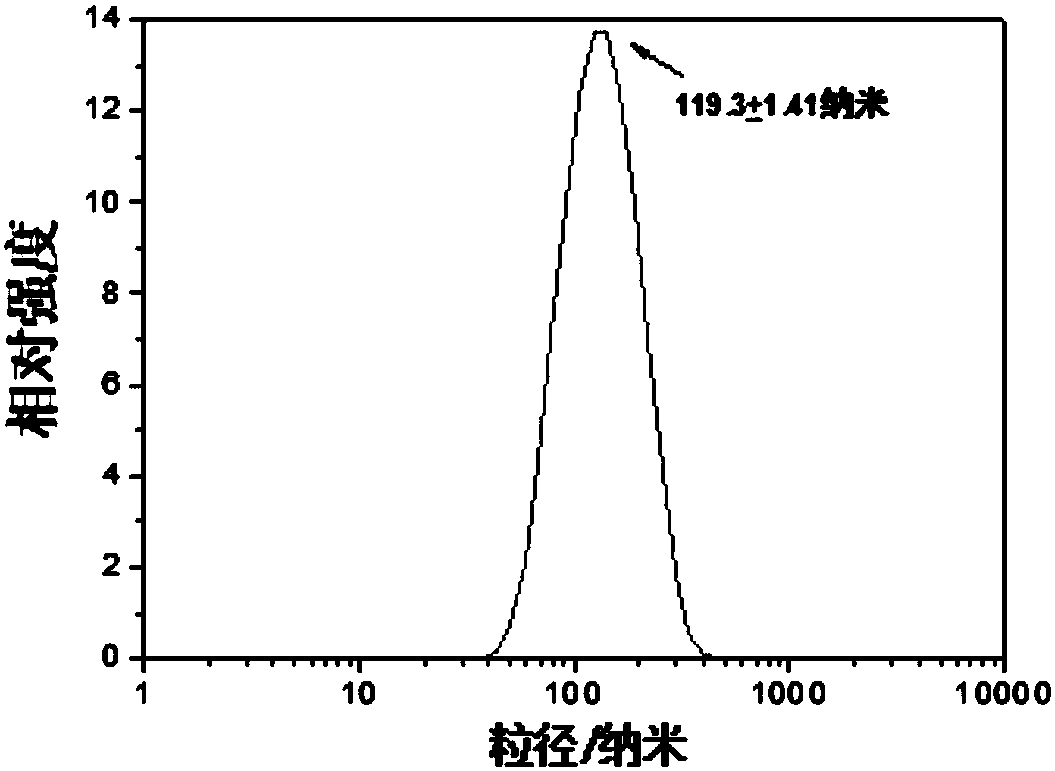
Fullerene polyaza ligand transition metal complex for magnetic resonance imaging and photodynamic therapy, preparation method, application and products
A technology of magnetic resonance imaging and photodynamic therapy, which is applied in photodynamic therapy, preparations for in vivo experiments, and medical preparations containing active ingredients, etc., which can solve the problems of low relaxation rate and poor magnetic resonance imaging effect. , to achieve strong phototoxicity, high-efficiency magnetic resonance effect, and the effect of reducing oxidative damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] When F (Fullerene) is C 70 [C(COOH) 2 ] 3 , M is gadolinium (Gd), when n=122, the preparation method of the fullerene polyaza ligand transition metal complex of formula 1-1 comprises:
[0058] (1) 0.12 millimolar of Boc-NH-PEG(2000)-COOH (purchased from Shanghai Yanyi Biotechnology Co., Ltd.), 0.15 millimolar of 2-(7-benzotriazole oxide)-N,N , N', N'-tetramethyluronium hexafluorophosphate (purchased from Alfa; as amidation reaction catalyst), 0.4 millimolar N, N-diisopropylethylamine (purchased from Enoch; as bound Acid agent) was dissolved in 15 ml of N,N-dimethylformamide solvent (purchased from Bailingwei; as an acid-binding agent), protected under argon atmosphere, stirred at 0°C for 30 minutes, and then added 0.1 mmol of 1, 4,7,10-tetraazacyclododecane-1,4,7-tri-tert-butyl triacetate (purchased from Alfa), stirred at room temperature for 24 hours, precipitated in anhydrous ether under ice bath conditions, dried in vacuo , to obtain the compound shown in formula...
Embodiment 2
[0068] The magnetic resonance performance test of the fullerene polyaza ligand transition metal complex obtained in Example 1, the steps and results are as follows:
[0069] (1) Prepare the aqueous solution (a) of the fullerene multi-aza ligand transition metal complex obtained in Example 1 with different concentrations as follows and the fullerene multi-aza ligand transition metal obtained in Example 1 with different concentrations as follows The physiological saline solution (b) of the complex is placed under a magnetic field strength of 0.5T, and the T 1 Its relaxation rate is calculated, and magnetic resonance imaging images are taken. Among them, T 1 is the longitudinal relaxation time, at the same concentration, T 1 The lower the value, the higher the relaxation rate and the better the MRI.
[0070] The aqueous solution (a) (Gd element concentration: 0.0132, 0.0264, 0.0528, 0.1056, 0.2112 mmol / liter) of the fullerene polyaza ligand transition metal complex obtained in...
Embodiment 4
[0077] Dark toxicity test is carried out to the fullerene polyaza ligand transition metal complex obtained in Example 1, the steps and results are as follows:
[0078] (1) Resuscitate A549 cells (Cell Resource Center, Shanghai Institute of Biology, Chinese Academy of Sciences), and adjust the cell density to 5×10 when the cells enter the logarithmic phase and grow stably. 4 / ml, seeded into 96-well plate, 37°C, 5% CO 2 , incubated for 24 hours;
[0079] (2) After the cells adhere to the wall, replace the DMEM solution (Corning R10-013-CV, purchasing company: Baierdi) with 200 microliters containing C 70 [C(COOH) 2 ] 3 The DMEM solution of the fullerene polyazaligand transition metal complex obtained in Example 1 with effective concentrations of 0, 3.6, 10.8, 18.0, 25.2, and 36.0 micromole / liter respectively, at 37° C., 5% CO 2 , incubated for 24 hours;
[0080] (3) Discard the DMEM solution containing the fullerene polyazaligand transition metal complex obtained in Exampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relaxation rate | aaaaa | aaaaa |
| Relaxation rate | aaaaa | aaaaa |
| Relaxation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com