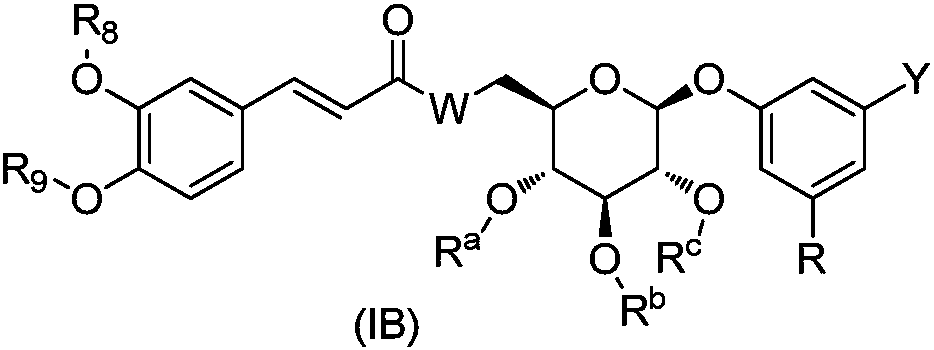
O-aryl glycoside derivative, pharmaceutical composition and application thereof
A drug and compound technology, applied in O-aryl glycoside derivatives, its pharmaceutical composition and application fields, can solve the problems of limited time window, poor effect, aggravated nerve damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: the synthesis of compound 1-1
[0112]
[0113] Step 1: Synthesis of Compound 1.1
[0114] Under ice bath, orcinol (10g, 82.6mmol) and imidazole (6.03g, 80.6mmol) were dissolved in dichloromethane (250mL), and tert-butyldimethylsilyl chloride (12.1g, 80.6mmol) was added dropwise . The reaction was then stirred overnight at room temperature. TLC monitoring showed that after the raw materials were reacted, the reaction solution was washed with water (50 mL), dried over anhydrous sodium sulfate, and concentrated. The crude product was purified by flash column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain compound 1.1 (8.8 g, yield 46%) as a colorless oil.
[0115] Step 2: Synthesis of Compound 1.2
[0116] Under ice-cooling, ferulic acid (3 g, 15.5 mmol) was dissolved in pyridine (5 mL), and acetic anhydride (4.5 mL) was added dropwise. After the dropwise addition, the reaction system was stirred at room temperature for 3 hours. Th...
Embodiment 2
[0125] Embodiment 2: the synthesis of compound 1-2
[0126]
[0127] Step 1: Synthesis of Compound 2.1
[0128] Compound 1.3 (1 g, 1.76 mmol) and sodium methoxide (95 mg, 1.76 mmol) were dissolved in methanol (50 mL), and the reaction system was stirred at room temperature for 2 hours. The reaction solution was acidified to pH=6 with acetic acid and spin-dried. The crude product was purified by flash column chromatography (dichloromethane:methanol=15:1) to obtain compound 2.1 (600 mg, yield 89%) as a white solid.
[0129] Step 2: Synthesis of compound 2.2
[0130] To a solution of compound 1.2 (440 mg, 1.0 mmol) in pyridine (10 mL) was added dropwise a solution of compound 2.1 in toluene (256 mg, 1.0 mmol, 30 mL). The reaction system was stirred overnight at room temperature and concentrated directly. The residue was purified by flash column chromatography (dichloromethane:methanol=15:1) to obtain compound 2.2 (230 mg, yield 37%) as a white solid.
[0131] Step 3: Synth...
Embodiment 3
[0135] Embodiment 3: the synthesis of compound 1-3
[0136]
[0137] Step 1: Synthesis of Compound 3.1
[0138] Potassium carbonate (33 g, 0.24 mol) and benzyl bromide (15.4 g, 0.09 mol) were added to orcinol (10 g, 0.08 mol) in acetonitrile (250 mL), and the reaction system was stirred at 80° C. overnight. The reaction system was filtered, and the filtrate was spin-dried. The crude product was purified by flash column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain compound 3.1 (6.5 g, yield: 38%) as a yellow oil.
[0139] Step 2: Synthesis of compound 3.2
[0140] To a solution of D-glucose pentaacetate (11.7 g, 0.03 mmol) and compound 3.1 (6.5 g, 0.03 mol) in dichloromethane (50 mL) was added boron trifluoride diethyl ether (4.3 g, 0.03 mol) dropwise. The reaction was stirred overnight at room temperature. The reaction solution was adjusted to pH=6 with aqueous sodium hydroxide solution (1M) and concentrated. The crude product was purified by flash c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com