Compound, palladium compound and nickel compound as well as preparation methods thereof
A technology of palladium compounds and compounds, applied in nickel organic compounds, chemical instruments and methods, compounds of group 5/15 elements of the periodic table, etc., can solve problems such as low activity, low copolymer molecular weight, low insertion ratio, etc., and achieve Effects of high activity, high copolymer molecular weight, and high insertion ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
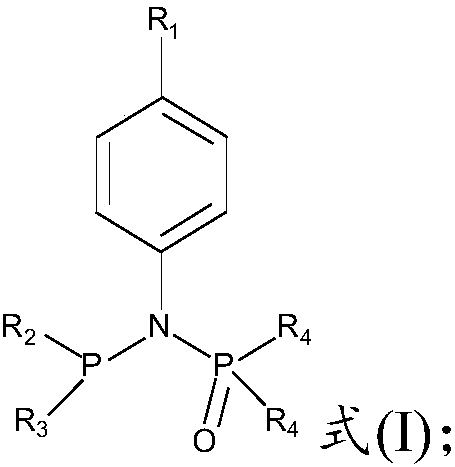
[0060] The present invention provides a kind of preparation method of the compound of formula (I) structure, comprising:
[0061] The compound of formula (II) is reacted with the compound of formula (III) to obtain the compound of formula (IV);
[0062] The compound of formula (IV) is reacted with the compound of formula (V) to obtain the compound of formula (I);
[0063]
[0064] where R 1 One or more selected from H, C1-C6 alkoxy, substituted C1-C6 alkyl, C1-C6 alkyl, dimethylamino, nitro, and halogen; the substitution of the substituted alkyl The base is fluorine;
[0065] R 2 , R 3 independently selected from substituted phenyl or substituted biphenyl; the substituent of the substituted phenyl is selected from one or more of C1-C6 alkoxy and C1-C10 alkyl; the substituted biphenyl The substituent of the group is selected from one or more of C1-C6 alkoxy and C1-C10 alkyl;
[0066] R 4 It is selected from C1-C6 alkyl group and C6-C12 aryl group.
[0067] Preferably...
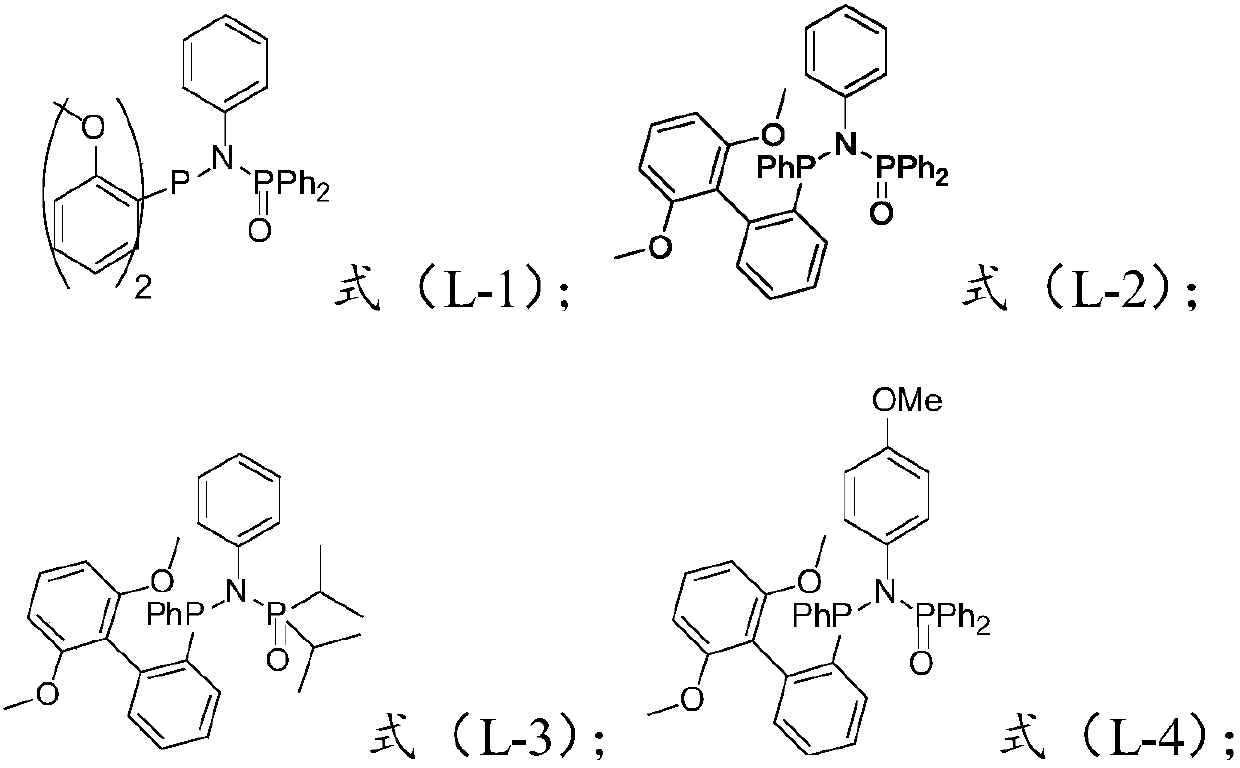
Embodiment 1
[0134] Example 1 Preparation of N-((2-methoxyphenyl)-12-phosphoranyl)-N,P,P-triphenylphosphine amide
[0135]
[0136] Dissolve aniline (0.9 g, 10.0 mmol) in 100 ml of EtO at -78°C 2 Slowly add an equivalent to the solution n BuLi (2.5M in hexane, 4.4mL, 1.1eq) was stirred for 15min, reacted at room temperature for 1-2h, and an equivalent of compound Ph was added to it at -78°C 2 POCl (2.4 g, 10.0 mmol) was reacted at room temperature for 12 h, and the target product A was obtained by column chromatography (where A does not need to be characterized, and can be directly used as a raw material in the next step). To a solution of dissolved A (2.925 g, 10 mmol) in 100 ml of THF at 0°C was addedn BuLi (2.5M in hexane, 4.4mL, 1.1eq) was reacted at room temperature overnight, compound B (3.08g, 1eq) was added at -78°C, the reaction was carried out for 24h, and the target product formula L-1 was obtained by column chromatography. Use a rotary evaporator to spin dry, add DCM to di...
Embodiment 2
[0138] Example 2 N-((2',6'-dimethoxy-[1,1'-biphenyl]-2-yl)(phenyl)phosphono)-N,P,P-triphenylene Preparation of phosphonamides
[0139]
[0140] The steps were the same as above, aniline was added, and ArPhPCl (3.6 g, 10.0 mmol) was added in the fourth step to obtain a white solid (3.5 g, 65%).
[0141] 1 H NMR (400MHz, CDCl 3 ):δ8.00(dd,J=11.8,7.1Hz,4H),7.34(m,8H),7.22(t,J=7.7Hz,2H),7.03(d,J=7.2Hz,2H),6.83 (t, J=7.4Hz, 2H), 6.78 (dd, J=10.8, 5.3Hz, 3H), 6.67 (dd, J=8.0, 5.2Hz, 2H), 3.68 (s, 6H). 13 C NMR (101MHz, CDCl3): δ160.7(d, J PC =18.0Hz)141.5(d,J PC =4.0Hz),134.0(d,J PC =3.0Hz),133.7(d,J PC =3.0Hz),133.3(d,J PC =3.0Hz)133.2(d,J PC =3.0Hz),131.7(d,J PC = 3.0Hz), 130.9(s), 129.9(d, J PC =4.0Hz),128.1(d,J PC =3.0Hz), 128.0(s), 125.5(s), 124.6(d, J PC =4.0Hz)124.4(d,J PC = 4.0Hz), 120.6(s), 110.1(s), 55.6(s). 31 P NMR (CDCl 3 ,162MHz):δ45.7(d,J=75.3Hz),28.3(d,J=75.3Hz).ESI-MS(m / z):[M+H] + Calcd for C 32 H 30 NO 3 P 2 , 538.1701; Found: 538.1700.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com