Fasudil impurity I preparation method
A technology of fasudil and impurity, which is applied in the field of preparation of fasudil impurity Ⅰ, which can solve the problems of scarce suppliers, high sales price, difficulty in obtaining fasudil impurity Ⅰ, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 acyl hydroxylamine
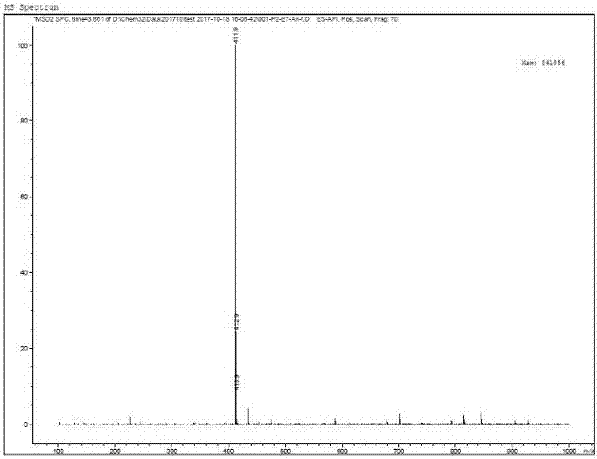
[0030] Fasudil (2.91g, 10mmol, 1.0eq), dipotassium hydrogen phosphate (2.91g, 12mmol, 1.2eq) and anhydrous DMF (30mL) were added to a 100mL single-necked bottle, and the reaction was stirred at 25°C for 3 hours. TLC monitoring fasudil reaction is complete. Add 60mL of water to the reaction flask, then extract with 50mL of ethyl acetate, and separate the layers. The aqueous phase was extracted twice more with 30 mL of ethyl acetate. The organic phases were combined, washed twice with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a white solid with a purity of 98%, which was acyl hydroxylamine, which was directly used in the next reaction.
[0031] MS(m / z): 411.9(M+H)+
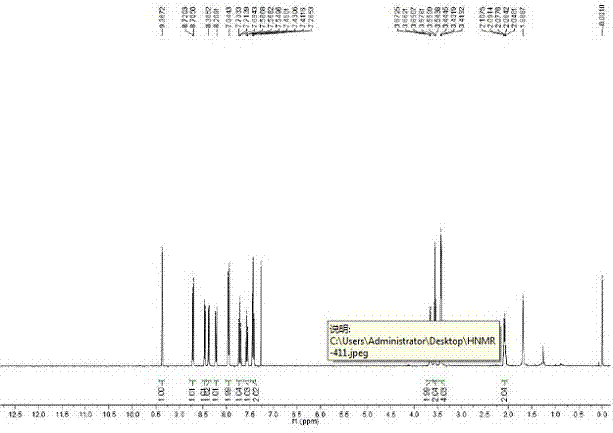
[0032] 1H NMR (400MHz, CDCl3): δ9.37(s, 1H), 8.71(d, J=6.12Hz, 1H), 8.46(d, J=6.16Hz, 1H), 8.38(dd, J=7.4Hz, 1Hz, 1H), 8.21(d, J=8.16Hz, 1H), 7.95(d, J=7.2Hz, 2H), 7.71(t, J=7.76Hz, 1H), 7.57(t, J=7.44Hz...
Embodiment 2
[0033] The preparation of embodiment 2 fasudil impurity I
[0034] Add tetrahydrofuran (40mL) to the spin-dried bottle of acylhydroxylamine above, stir to dissolve, control the reaction temperature at 0-10°C, add dropwise a solution of lithium hydroxide (264mg, 1.1mmol, 1.1eq) dissolved in water (8mL), After the dropwise addition was completed, the reaction was carried out overnight, and the reaction solution was concentrated to dryness. The crude product was purified by column chromatography to obtain 2.80 g of a white solid with a purity of 99% and a yield of 91%, which was Fasudil Impurity I.
[0035] MS(m / z): 308(M+H)+
[0036] 1H NMR (400MHz, DMSO-d6): δ9.50(s, 1H), 8.71(d, J=6.12Hz, 1H), 8.47(d, J=8.24Hz, 1H), 8.35(d, J=6.12 Hz, 1H), 8.33(dd, J=7.36, 1Hz, 1H), 8.08(s, 1H), 7.85(t, J=7.56Hz, 1H), 3.49-3.50(m, 2H), 3.42(t, J=6.24Hz, 2H), 2.87-2.91(m, 4H), 2.03-1.91(m, 2H).
Embodiment 3
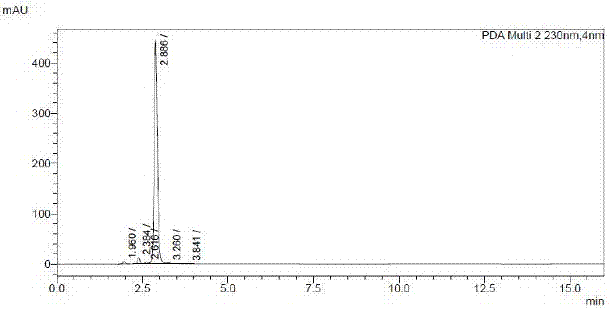
[0037] The HPLC detection of embodiment 3 fasudil impurity I
[0038] The HPLC detection method of Fashur impurity Ⅰ is as follows:
[0039] Chromatographic column: InertSustain C18 4.6X250mm 5μm
[0040] mobile phase:
[0041] A: methanol: 60% B: 1.4g / L disodium hydrogen phosphate
[0042] Mobile phase ratio: A:B=70:30
[0043] Column temperature: 35°C
[0044] Detection wavelength: 230nm
[0045] Flow rate: 1.2mL / min
[0046] Time: 16.00min
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com