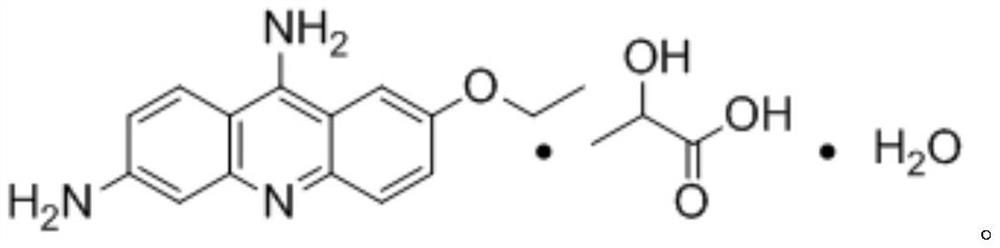
A kind of preparation technology of ethacridine lactate
A technology for preparing ethacridine lactate and its preparation technology, which is applied in the field of preparation technology of ethacridine lactate and can solve problems such as difficulty in adapting to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation process of ethacridine lactate, comprising:
[0029] First add 10g of 2-ethoxy-6-nitro-9-aminoacridine, 8g of zinc powder, and 3.9g of 90% lactic acid into the 500mL reaction bottle;
[0030] Then add 150mL of ethanol, heat to 80°C for 5 hours under the protection of nitrogen, and then filter while it is hot. The filtrate is naturally cooled to room temperature to precipitate a bright yellow solid. The bright yellow solid is ethacridine lactate solid, with a weight of 8.5g, the total yield is 70%, and the purity is 99.52%.
Embodiment 2
[0032] A preparation process of ethacridine lactate, comprising:
[0033] First add 200g of 2-ethoxy-6-nitro-9-aminoacridine, 160g of zinc powder, and 78g of 90% lactic acid into the 5L reaction flask;
[0034] Then add 3L of ethanol, heat to 80°C for 6 hours under the protection of nitrogen, and then filter while it is hot. The filtrate is naturally cooled to room temperature to precipitate a bright yellow solid. The bright yellow solid is ethacridine lactate solid, with a weight of 174g, the total yield is 71.6%, and the purity is 99.36%.
Embodiment 3
[0036] A preparation process of ethacridine lactate, comprising:
[0037] First add 2kg of 2-ethoxy-6-nitro-9-aminoacridine, 1.6kg of zinc powder, and 0.78kg of 90% lactic acid into the 50L reactor;
[0038] Then add 30L of ethanol, heat to 80°C for 7 hours under the protection of nitrogen, and then filter while it is hot. The filtrate is naturally cooled to room temperature to precipitate a bright yellow solid. The bright yellow solid is the ethacridine lactate solid, with a weight of 1.738g, the total yield is 71.4%, and the purity is 99.34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com