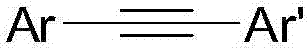
Preparation method of diaryl acetylene compounds
A diaryl acetylene and compound technology, applied in the field of preparation of organic compounds, can solve the problems of few applicable objects, low yield, and side reactions in reductive coupling reactions, and achieve variable structure, convenient synthesis, and mild synthesis conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] (1) Preparation of raw materials
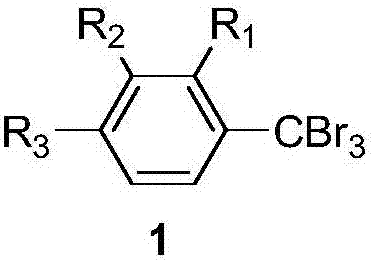
[0046] All gem-tribromomethyl arene compounds can be prepared by existing common synthesis methods, and the molecular structure of the gem-tribromomethyl arene compounds used in the examples is shown in the following formula.
[0047]
[0048] Raw compound abbreviation
R 1
R 2
R 3
1a
H-
H-
H-
1b
F-
H-
H-
1c
Cl-
H-
H-
1d
Br-
H-
H-
1e
H-
Cl-
H-
1f
H-
Br-
H-
1g
H-
CH 3 O-
H-
1h
H-
CH 3 C(O)-
H-
1i
H-
H-
F-
1j
H-
H-
Cl-
1k
H-
H-
Br-
1l
H-
H-
tert-C 4 h 9 -
1m
H-
H-
-CF 3
1n
H-
H-
-CN
1p
H-
H-
CH 3 C(O)-
1q
H-
H-
CH 3 OC(O)-
1r
H-
H-
-NO 2
[0049] (2) Synthesis method
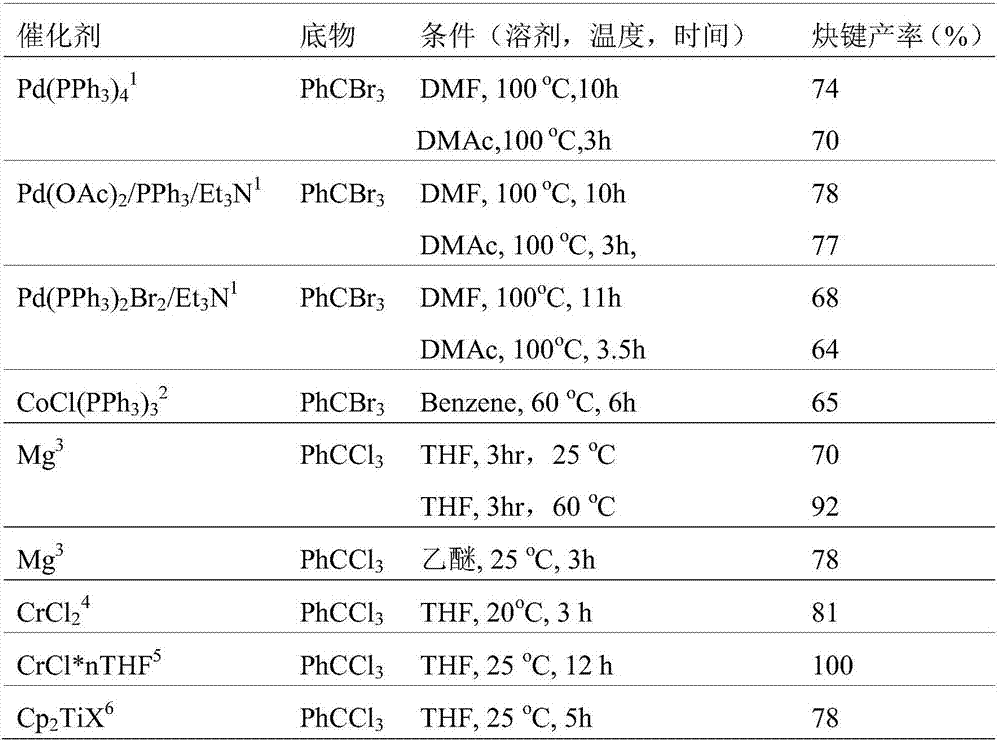
[0050] Deoxidize a certain stoichiometric ratio of gem-tribromomethyl...
Embodiment 1 3
[0057] The synthesis of embodiment 1 tribromotoluene (1a)
[0058] Add 9.2 grams of toluene (0.1mol), 58.7 grams of NBS (0.33mol), 4.0 grams of BPO (0.017mol) and 300mL of CCl in a 500mL three-necked flask 4 , through nitrogen for 10 minutes. Heated to reflux, and stopped the reaction after 5 hours of reaction. The filtrate obtained after filtration was concentrated to obtain the crude product. The pure product 20.7 g of 1a was obtained by flash chromatography (petroleum ether). Yield 63%. 1 H NMR (400MHz, CDCl 3 ):δ8.03-8.00(m,2H),7.43-7.38(m,2H),7.36-7.32(m,1H). 13 C NMR (101MHz, CDCl 3 ):δ147.07,130.25,128.18,126.61,36.45.GC-MS(EI,m / z):249[M-Br] + .Anal.Calcd.for C 7 h 5 Br 3 : C, 25.57; H, 1.53. Found: C, 25.51; H, 1.51.
Embodiment 2
[0060] Example 2 Synthesis of o-fluorobenzotribromotoluene (1b), using o-fluorotoluene as raw material, yield 76%. 1 H NMR (400MHz, CDCl 3 ,ppm):δ6.85-6.91(m,2H),7.04-7.31(m,2H), 13 C NMR (101MHz, CDCl 3 ): δ30.93,117.31,123.24,128.44,132.73,133.40,159.19.GC-MS(EI,m / z):346[M + ].Anal.Calcd.for C 7 h 4 Br 3 F: C, 24.24; H, 1.16. Found: C, 24.20; H, 1.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com