Immunohistochemical detection kit used for prognosis after hepatectomy
A detection kit and immunohistochemical technology, applied in the biological field, can solve the problems of ignoring the prognosis of myeloid cells and the important impact of anti-tumor treatment, failing to fully reflect the actual situation, and poor accuracy, so as to achieve mature test methods and ensure The effect of accuracy and simplicity of the detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Screening of antibodies used in the immunohistochemical detection kit of the present invention
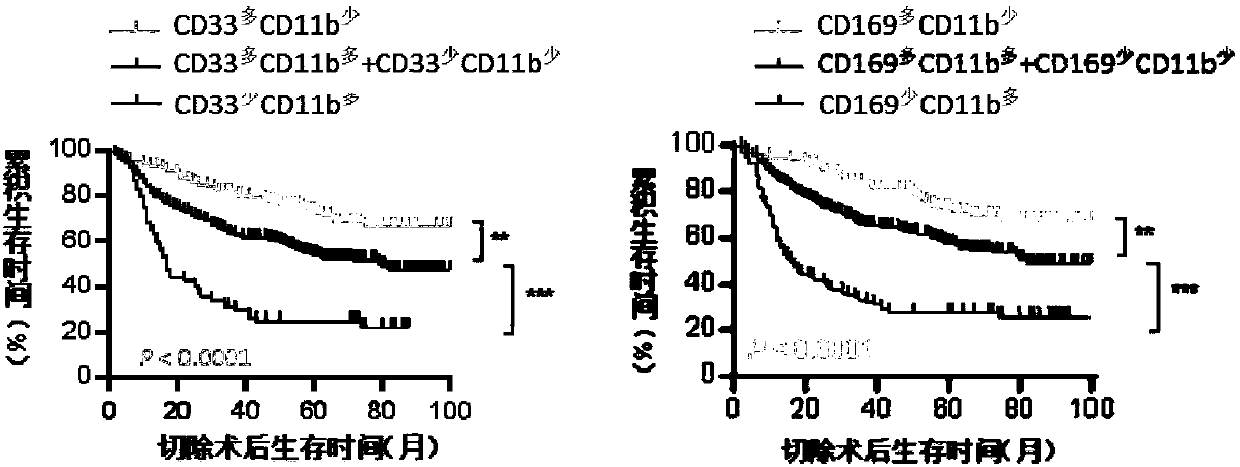
[0020] The present invention selects three protein molecular markers of myeloid immune cells (CD169, CD33, CD11b), and these three markers have independent predictive ability for patient prognosis. Among them, CD169 is used to mark CD169 + of myeloid cells, CD33 was used to label CD33 + myeloid cells, CD11b is used to mark CD11b + of myeloid cells.
[0021] Table 1. Sources of antibodies and specific experimental conditions
[0022] Mark
Antibody source
Color development time
CD33
Leica, Cat. No.: NCL-L-CD33
High pressure 10min, EDTA (pH9.0)
10min
CD11b
Abcam, Item No.: ab52478
High pressure for 10min, 10mMCB (pH6.0)
0.5min
CD169
R&D, Item No.: AF5197
Microwave 20min, EDTA (pH9.5)
5min
[0023] EDTA: ethylenediaminetetraacetic acid; CB: citrate buffer.
[0024] Th...
Embodiment 2
[0030] Embodiment 2: The double-antibody and triple-antibody combination scheme of the immunohistochemical detection kit of the present invention
[0031] The preparation method of the immunohistochemical detection kit of the present invention is the same as that of conventional immunohistochemical detection kits, except that the protein molecules detected by the first antibody used in the kit are: CD33, CD11b, and CD169. These three primary antibodies can detect clear positive signals in paraffin sections of liver cancer tissues.
[0032] For the immunohistochemical detection kit described in the present invention, its effectiveness is tested through the following steps.
[0033] (1) Specimen testing
[0034] 1. Select 465 cases of liver cancer tissue wax blocks containing tumor areas, and ensure that there is no large area of necrosis;
[0035] 2. After selecting points for each sample of wax blocks, arrange them into a chip array, obtain 4 μm tissue chip paraffin sectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com