Method for separating and analyzing avenanthramide D and dihydroavenanthramide D
A technology for separation and analysis of avenanthramide, which is applied in the field of chemical substance analysis and separation, can solve problems such as small difference and achieve the effect of a reliable method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
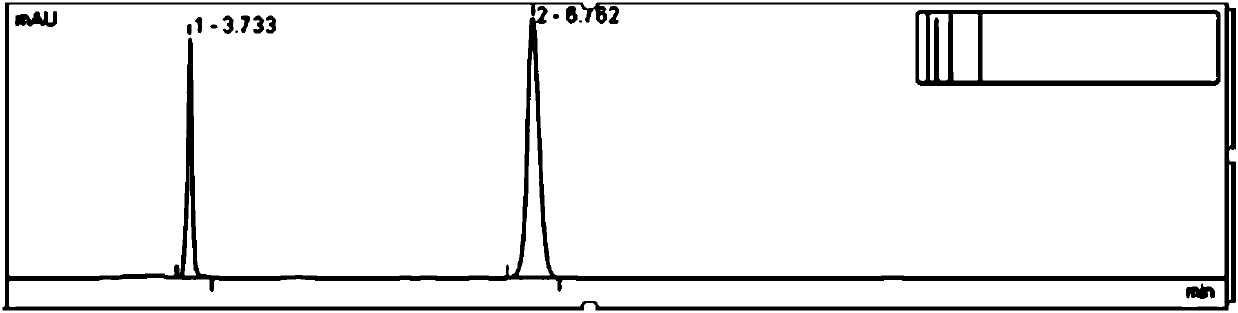
Embodiment 1
[0024] Instrument: ULtimate 3000 high performance liquid chromatography; UV detector
[0025] Chromatographic column: chiral HDL column (β-cyclodextrin as stationary phase)
[0026] Mobile phase: main mobile phase: 30% acetonitrile, 70% water (0.3% triethylamine, 0.3% glacial acetic acid), the water of 560mL, the acetonitrile of 240mL, the triethylamine of 1.7mL, the glacial acetic acid of 1.7mL are mixed, Ultrasonic degassing for 30min. Placed in the chromatographic inlet;
[0027] Mobile phase flow rate: 0.8mL / min
[0028] Detection wavelength: 254nm
[0029] Column temperature: 30°C
[0030] Injection volume: 0.8uL
[0031] Test procedure: Weigh 10mg of oat alkaloid raw material powder, put it in a 50mL measuring bottle, add ethanol to dissolve and dilute to the mark, shake well, take 20mL precisely, put it in a 25mL sample bottle, shake well, as the raw material sample solution, place in the autosampler tray.
[0032] Set the detection method and sequence: the autom...
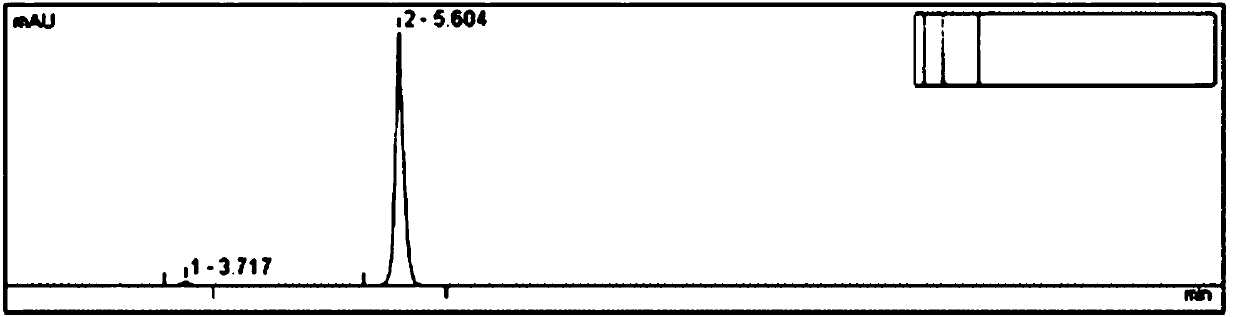
Embodiment 2
[0034] Instrument: ULtimate 3000 high performance liquid chromatography; UV detector
[0035] Chromatographic column: chiral HDL column (β-cyclodextrin as stationary phase)
[0036] Mobile phase: main mobile phase: 30% acetonitrile, 70% water (0.3% triethylamine, 0.3% glacial acetic acid), the water of 560mL, the acetonitrile of 240mL, the triethylamine of 1.7mL, the glacial acetic acid of 1.7mL are mixed, Ultrasonic degassing for 30min. Placed in the chromatographic inlet.
[0037] Flow rate: 0.8mL / min
[0038] Detection wavelength: 254nm
[0039] Column temperature: 30°C
[0040] Injection volume: 0.8uL
[0041] Test procedure: Weigh 10 mg of standard dihydrooat alkaloid raw material powder, put it in a 50 mL measuring bottle, add ethanol to dissolve and dilute to the mark, shake well, accurately measure 20 mL, place it in a 25 mL sample bottle, shake well, and use it as a raw material Sample solution, placed in the autosampler tray.
[0042] Set the detection method ...
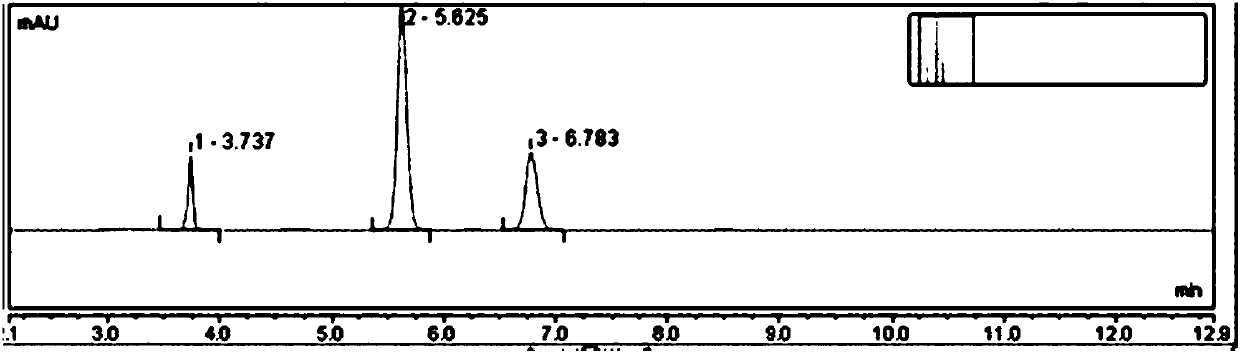
Embodiment 3
[0044] Instrument: ULtimate 3000 high performance liquid chromatography; UV detector
[0045] Chromatographic column: chiral HDL column (β-cyclodextrin as stationary phase)
[0046] Mobile phase: main mobile phase: 30% acetonitrile, 70% water (0.3% triethylamine, 0.3% glacial acetic acid), the water of 560mL, the acetonitrile of 240mL, the triethylamine of 1.7mL, the glacial acetic acid of 1.7mL are mixed, Ultrasonic degassing for 30min. Placed in the chromatographic inlet.
[0047] Flow rate: 0.8mL / min
[0048] Detection wavelength: 254nm
[0049] Column temperature: 30°C
[0050] Injection volume: 0.8uL
[0051] Test procedure: Weigh 10 mg of the mixed sample of avena alkaloid D and standard dihydroavenine D, put them together in a 50mL measuring bottle, add ethanol to dissolve and dilute to the mark, shake well, accurately measure 20mL and put it in a 25mL sample bottle, Shake well, and place it in the autosampler tray as the raw sample solution.
[0052] Set the det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com