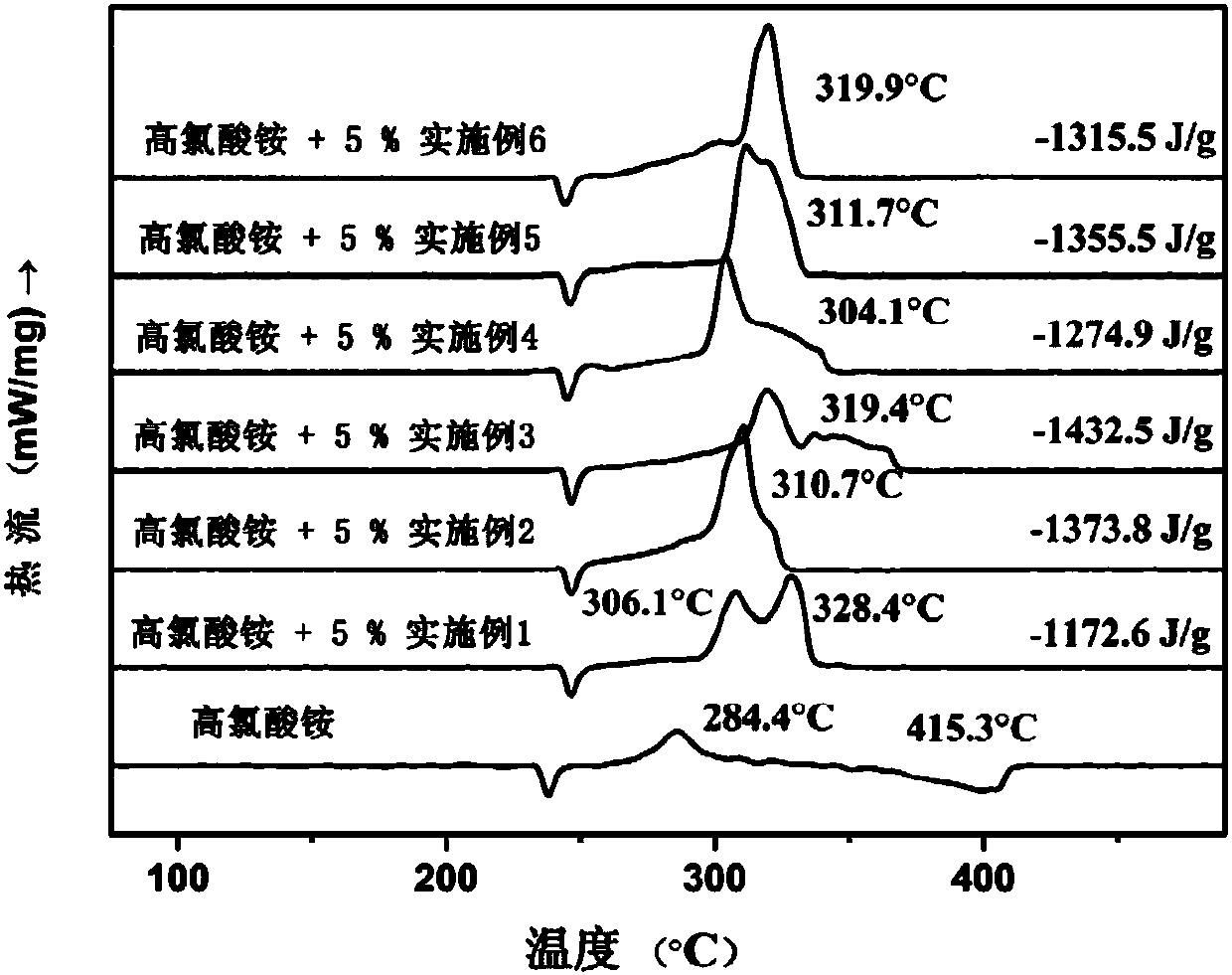
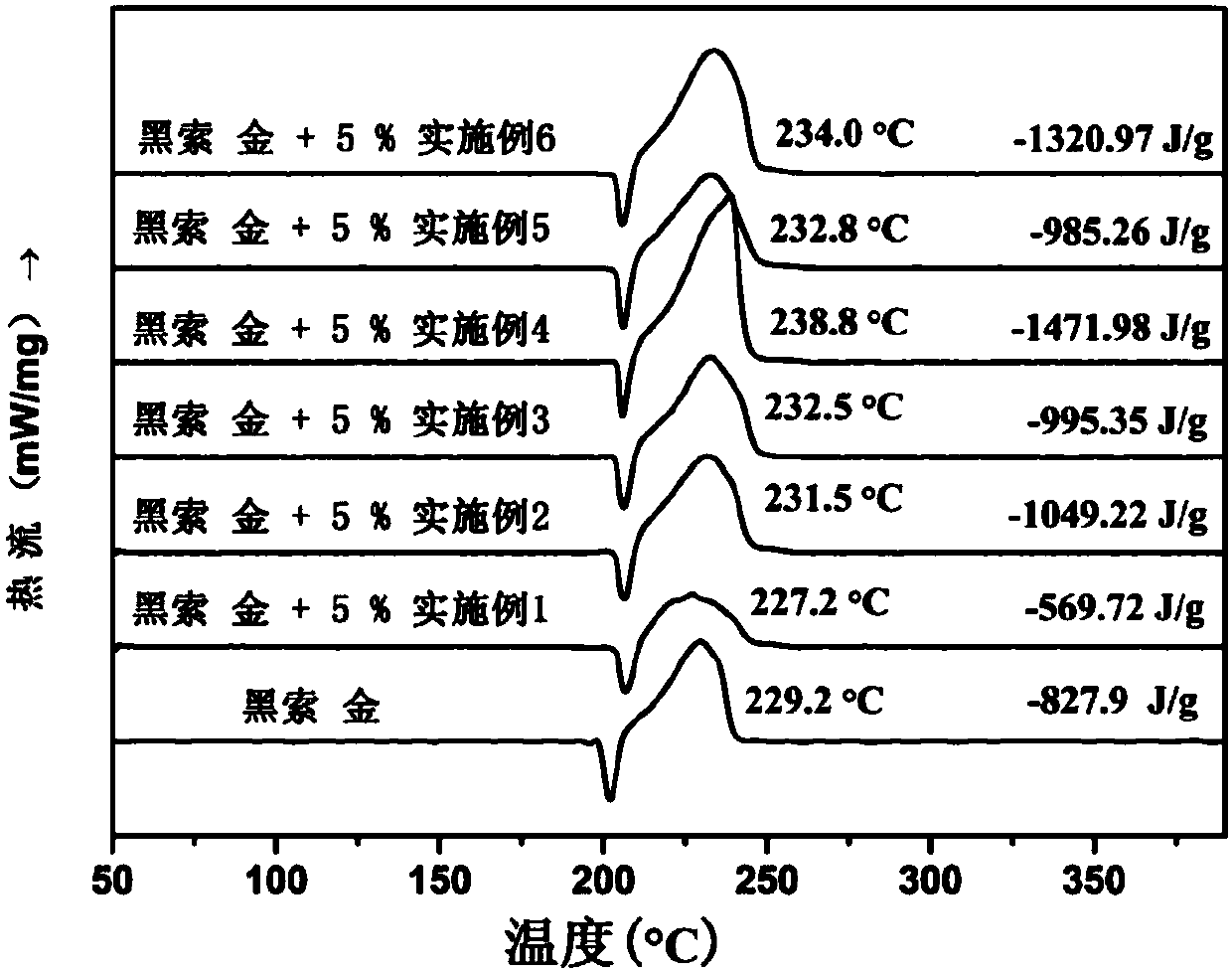
Ferrocene triazole ionic-type nitrogen-enriched energetic metal complex and preparation method thereof
A technology of ferrocene triazole and metal complexes, which is applied in the preparation of organic compounds, metallocenes, organic compounds/hydrides/coordination complex catalysts, etc. problems, to achieve the effects of good thermal stability, low vapor pressure and volatility, and overcoming the cumbersome synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 0.18g (0.5mmol) Zn(ClO 4 ) 2 ·6H 2 O was dissolved in a 100mL round bottom flask containing 10mL of distilled water, and when the temperature rose to 60℃, 0.28g (1.0mmol) of Ferrocene Triazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it. With 0.25g (1.0mmol) potassium tetracyanoacrylate solution dissolved in 20mL of distilled water, react for 3 hours, a brick red precipitate is formed, filter, and wash the filter cake with absolute ethanol and distilled water, at room temperature After vacuum drying, the ferrocene triazole ion type nitrogen-rich energetic metal complex with the following structural formula is obtained:
[0031]
[0032] The yield rate is 57%, and the structural characterization data is: IR(KBr,cm -1 ): 3320(w,br), 2194(m), 1617(s), 1555(vs), 1422(m), 1280(m), 1226(m), 1129(s), 1093(s), 810 (w),641(m),491(m)cm -1 ; Elemental analysis (theoretical calculated values in parentheses): C% 48.80 (48.77), H% 2.80 (2.76...
Embodiment 2
[0034] 0.18g (0.5mmol) Cu(ClO 4 ) 2 ·6H 2 O was dissolved in a 100mL round bottom flask containing 10mL of distilled water, and when the temperature rose to 60℃, 0.28g (1.0mmol) of Ferrocene Triazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it. With 0.25g (1.0mmol) sodium picrate solution dissolved in 20mL of distilled water, react for 3 hours, a green precipitate is formed, filter, and wash the filter cake with absolute ethanol and distilled water, and vacuum dry at room temperature to obtain ferrocene with the following structural formula Nitrogen-rich nitrogen-enriched metal complexes:
[0035]
[0036] The yield is 62%, and the structural characterization data is: IR (KBr, cm -1 ): 3435(m,br), 3081(m), 1652(vs), 1635(vs), 1555(vs), 1484(s), 1439(m), 1324(vs), 1174(m), 1085 (s),916(w),783(w),703(w),482(m)cm -1 ; Elemental analysis (theoretical calculation values in parentheses): C% 43.20 (42.22), H% 2.65 (2.7), N% 18.32 (18.14).
Embodiment 3
[0038] Add 0.18g (0.5mmol) Zn(ClO 4 ) 2 ·6H 2 O was dissolved in a 100mL round bottom flask containing 10mL of distilled water, and when the temperature rose to 60℃, 0.28g (1.0mmol) of Ferrocene Triazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it. With 0.25g (1.0mmol) sodium picrate solution dissolved in 20mL of distilled water, react for 3 hours, a green precipitate is formed, filter, and wash the filter cake with absolute ethanol and distilled water, and vacuum dry at room temperature to obtain ferrocene with the following structural formula Nitrogen-rich nitrogen-enriched metal complexes:
[0039]
[0040] The yield is 62%, and the structural characterization data is: IR (KBr, cm -1 ): 3329(w,br), 3307(w,br), 1626(vs), 1546(vs), 1431(w), 1315(m), 1271(vs), 1085(vs), 1022(vs) ,801(s),491(m)cm -1 ; Elemental analysis (theoretical calculation values in parentheses): C% 43.20 (42.15), H% 2.87 (2.7), N% 18.32 (18.11).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com