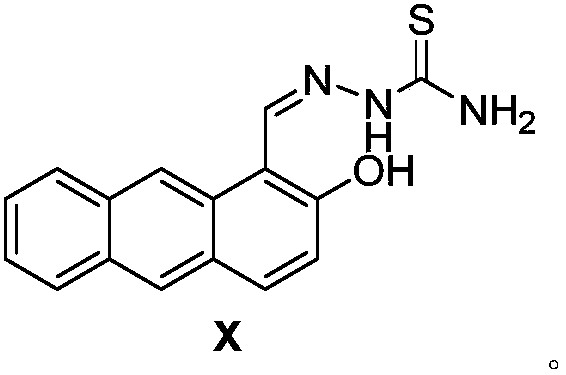
Anthracene derivative fluorescent probe as well as synthesis method and application of anthracene derivative fluorescent probe to H2S detection
A technology of fluorescent probes and anthracene derivatives, which is applied in the field of medical detection, can solve the problems of limited fluorescent probes, and achieve the effects of strong repeatability, mild experimental environment, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthetic method of anthracene derivative fluorescent probe NLN comprises the following steps:
[0022]
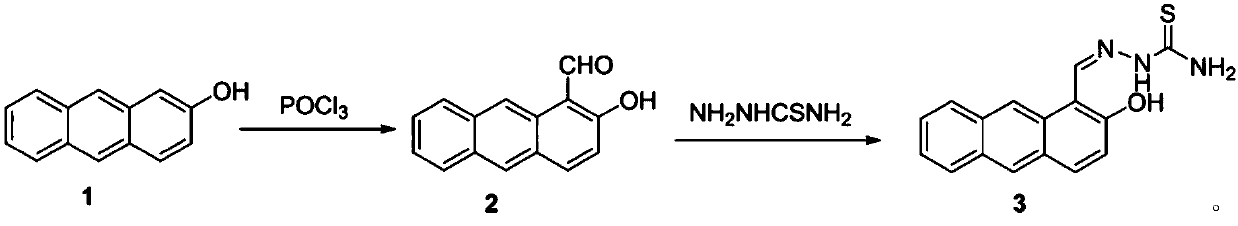
[0023] Step i. Phosphorus oxychloride was added dropwise to dimethylformamide under ice bath at 0°C, and then 2-hydroxyanthracene (5mmol) dissolved in dimethylformamide (5mmol) was added dropwise to the above mixture The reaction was gradually raised to room temperature and stirred for 2 hours, and then the reaction flask was transferred to a 60°C oil bath for 10 hours of reaction. After the reaction, the reaction solution was poured into ice water, neutralized with sodium carbonate to pH = 7, filtered, the solid was washed with cold ethanol and distilled water successively, and dried to obtain compound 2.
[0024] Step ii. Compound 2 (2 mmol) and compound thiosemicarbazide (2 mmol) were sequentially added into a 25 mL round bottom flask containing ethanol under stirring. Stirred and refluxed for 8 hours, filtered to obtain a solid crude product, and recryst...
Embodiment 2
[0028] In vitro test experiment of fluorescent probe NLN
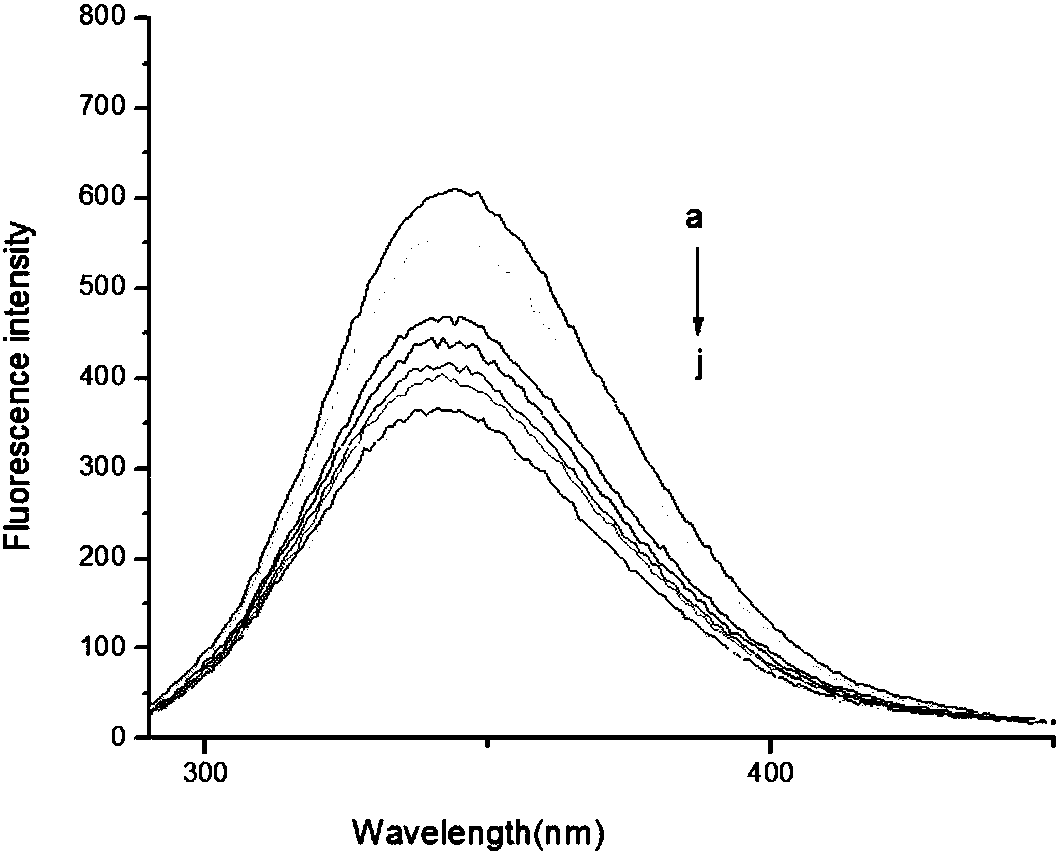
[0029] The fluorescent probe NLN synthesized in Example 1 was mixed with Cu in 20mM HEPES buffer (pH 7.4, 0.5%DMSO, 25°C 2+ The emission and absorption properties of the reacted solution were studied. Such as figure 1 As shown, 50 μM Cu was added to the NLN solution 2+ The absorption band at 300-400nm drops after giving the concentration range figure which cannot be seen. Cu was added to the NLN solution 2+ After the evaluation of the changes in the fluorescence emission spectrum of the solution, it can be obtained that the free fluorescent probe emits strong fluorescence under physiological pH conditions. –1 )a~j:0,1.0,2.0,3.0,4.0,5.0,6.0,7.0,8.0,9.0] added Cu 2+ After (1.0equiv), it will cause the fluorescence to be completely quenched, which means that the probe NLN reacts to Cu through the Turn-off mechanism 2+ identification was carried out where the pH value of the solution was 7.43 for NLN and Cu 2+ The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com