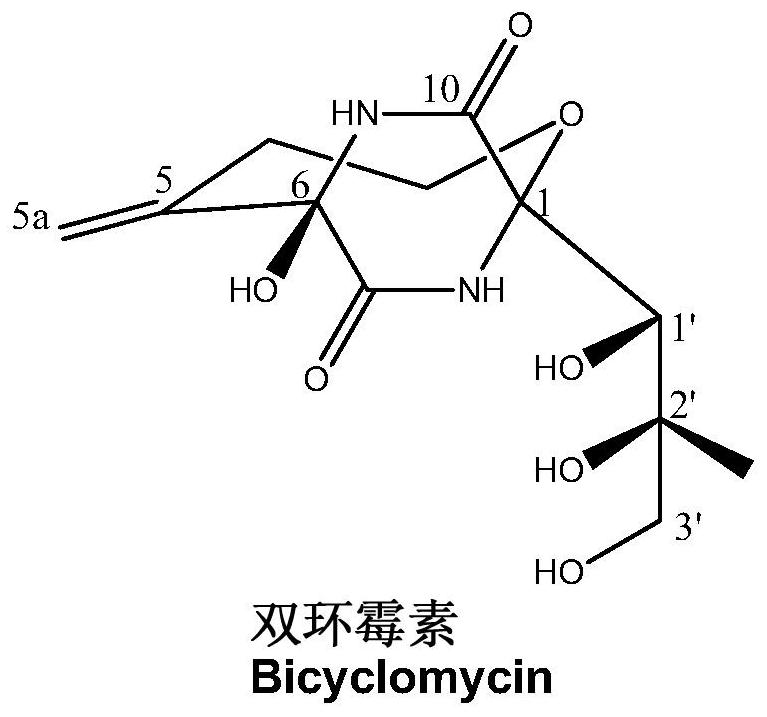
The function and application of oxidase in the biosynthesis of dicyclomycin
A technology of dicyclomycin and dioxygenase, applied in the field of biotechnology and engineering, can solve the problem of low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1. Whole Genome Sequencing Obtains the Bicyclomycin Biosynthetic Gene Cluster Sequence
[0137] (1) Extraction of S. sapporonensis ATCC 21532 genomic DNA
[0138] Inoculate 100 μL of S. sapporonensis ATCC 21532 spore suspension into 3 mL of TSB (TSB 30 g, 1 L) liquid medium, culture at 30° C., 250 rpm, and shake for about 24-36 hours, reaching the late logarithmic growth phase. Take 1mL and inoculate into 50mL TSB (containing 5mM MgCl 2 , 0.5% glycine), 30 ℃, 250rpm culture, about 24-36hr after reaching the early stage of the stable growth period, it was yellowish and turbid, and there were a lot of suspended mycelia. Centrifuge the bacterial solution at 4°C, 3500 rpm for 10 minutes to collect the mycelium, wash twice with lysis buffer to obtain about 2-4 mL of mycelium. Add 10 mL of SET lysis buffer (containing 5 mg / mL lysozyme) to 1 mL of mycelia, vortex until uniform, and incubate in a water bath at 37 °C for 30 min-60 min. Add 0.1mL proteinase K (10mg / mL,...
Embodiment 2
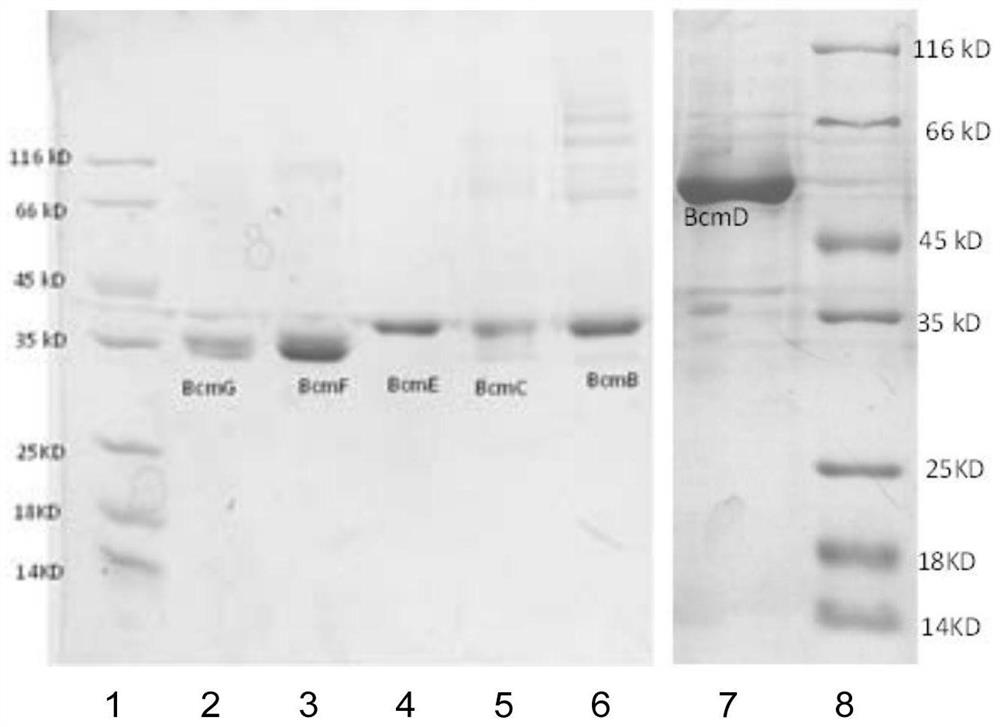
[0144] Example 2. Expression and purification of five α-ketoglutarate-dependent dioxygenases and one cytochrome P450 monooxygenase
[0145] Using the genomic DNA of the dicyclomycin-producing strain S. sapporonensis ATCC 21532 as a template, the PCR reaction system was composed of water, dNTP, DMSO, high-fidelity Primestar DNA polymerase and its buffer, and five α-ketoglutarate-dependent The genes encoding dioxygenases BcmB, BcmC, BcmE, BcmF, BcmG and one cytochrome P450 monooxygenase BcmD were amplified by PCR. The primer sequences for amplifying each gene are shown in Table 2. The 6 gene fragments were obtained by PCR cloning. After separation and purification by gel electrophoresis, restriction endonucleases HindIII and NdeI were added to digest them respectively, and they were respectively connected into pET28a treated with the same restriction enzymes to complete the construction of each protein expression vector. . Then each protein expression vector was transformed in...
Embodiment 3
[0150] Example 3. BcmE in vitro enzyme catalyzes cyclic dipeptide substrate cIL to generate II
[0151] (1) Synthesis and identification of cyclic dipeptide substrate cIL
[0152] The synthesis steps of cyclic dipeptide substrate cIL are as follows:
[0153] (a) Synthesis of condensation intermediate: H-Ile-Ome.HCl dissolved in CH 2 Cl 2 Add TEA and Boc-Leu-OH.H under ice-salt bath 2 O, add EDC.HCl and HOBT under ice-water bath. Stir in an ice-water bath for 2 hours, then stir overnight at room temperature. The reaction solution was saturated with NH 4 Cl solution was washed twice, the organic phase was washed once with saturated NaCl solution, the organic phase was separated, and anhydrous MgSO 4 dry. Filtration, rotary evaporation, wet silica gel column, ethyl acetate and petroleum ether were eluted in proportion. Spot the plate, develop the color with ammonium molybdate, and dry it with an alcohol lamp. The product was collected and spin-dried to obtain a condensat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com