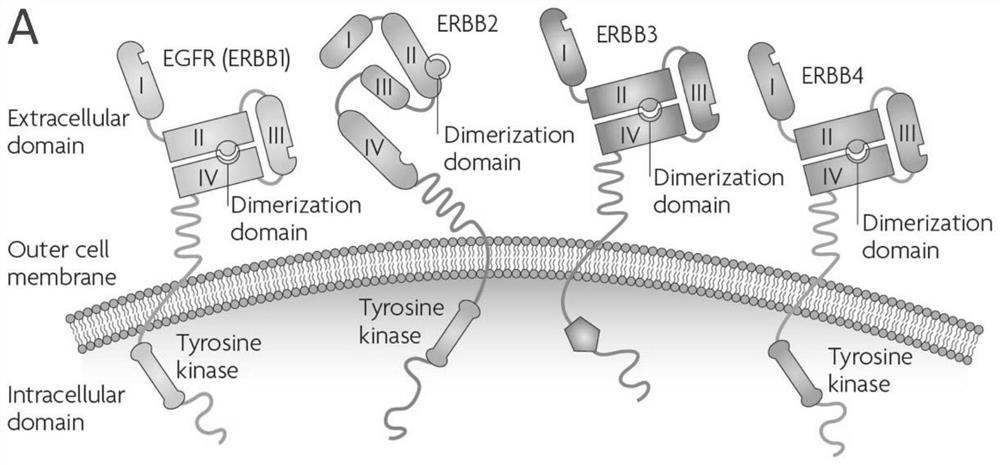
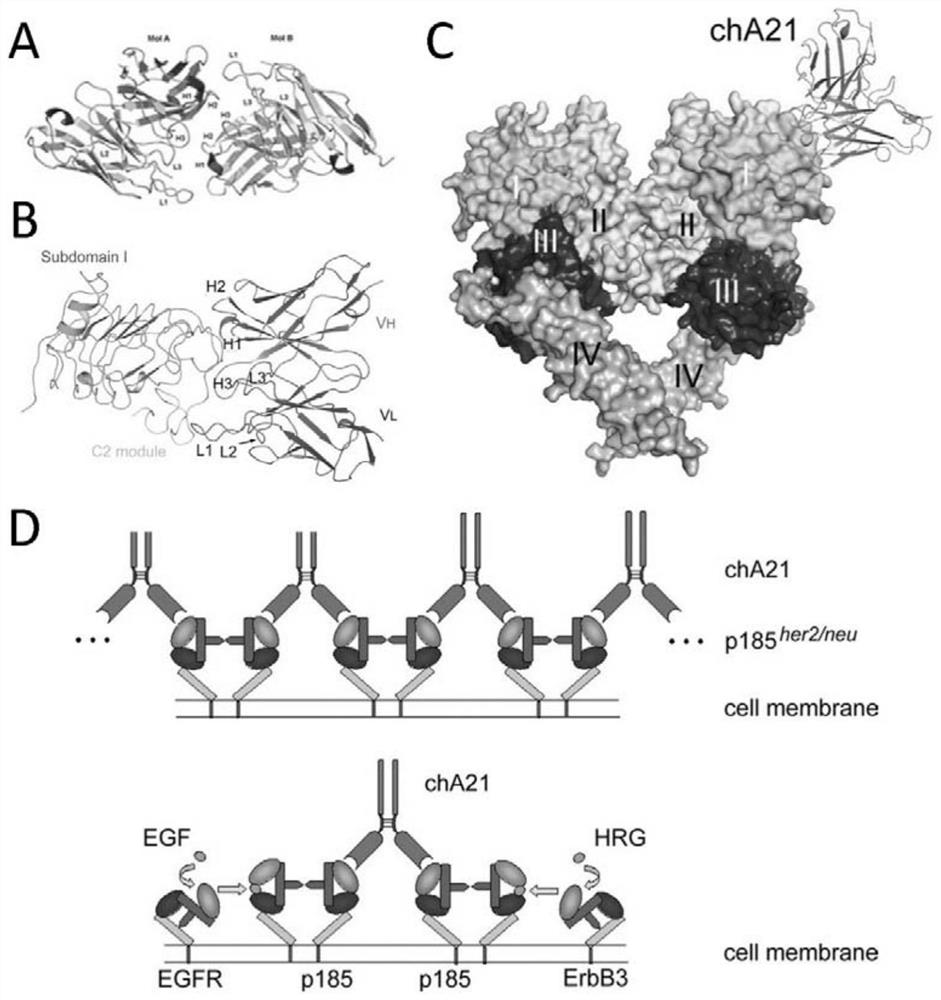
ERBB2 epitope peptide bound to E9 antibody
A technology of antibody complexes and antigens, applied in the field of biomedicine, can solve problems such as limiting the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1, Analysis of antigenic epitopes in the scFv-ECD complex
[0082] This example analyzes the scFv of the E9 antibody (that is, the single-chain antibody formed by connecting the light chain variable region and the heavy chain variable region of E9, referred to as E9 scFv) and the ECD region of the ErbB2 protein (ErbB2 ECD, the sequence in the sequence listing 6) The formed scFv-ECD complex determined the E9 antibody and ErbB2 ECD binding region and the corresponding epitope.
[0083] 1. Preparation of scFv-ECD complex
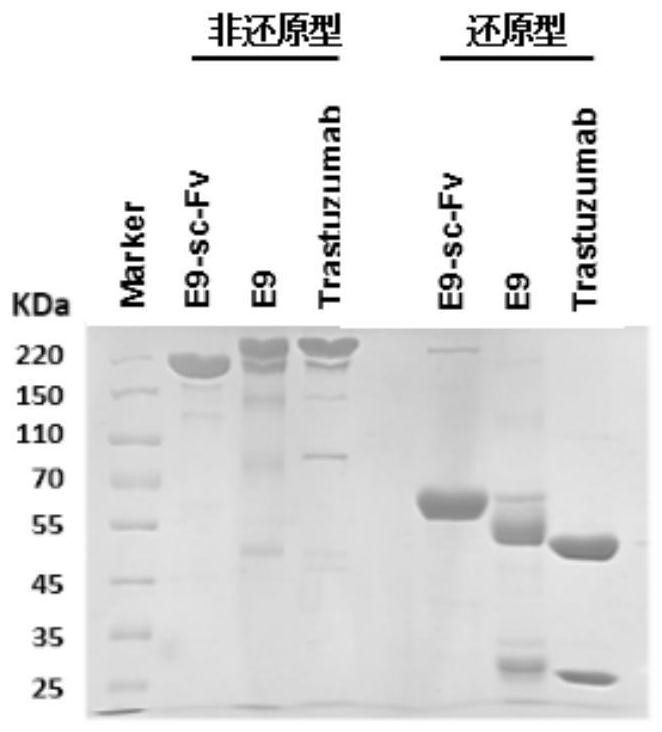
[0084] Both E9 scFv and ErbB2 ECD express an Fc fragment of IgG1 in series at their carboxy-terminal by genetic engineering methods, and add an enterokinase restriction site before the Fc fragments of the two (the resulting fusion proteins are respectively designated as E9 scFv- Fc and ErbB2 ECD-Fc) were prepared. The fusion proteins were all secreted and expressed by the eukaryotic expression system CHO-K1 cell line, purified and mixed at a mo...
Embodiment 2
[0157] Example 2, E9 antibody has the effect of inhibiting tumor cell growth
[0158] The test cells are: OE19 (Nanjing Kebo Biotechnology Co., Ltd.)
[0159] The antibodies or antibody combinations to be tested are: E9, Pertuzumab, Herceptin, Pertuzumab+Herceptin (mass ratio 1:1), E9+Herceptin (mass ratio 1:1).
[0160] Specific steps are as follows:
[0161] 1. Trypsinize the cells in logarithmic growth phase, add culture medium to make cell suspension;
[0162] 2. 3000-20000ug antibody / 100μl per well, add cells into 96-well plate, and culture for 24hr;
[0163] 3. From the highest concentration, add 100 μl of the test product diluted >9 concentrations by 2-4 times to each well, 3-6 parallel wells for each concentration, and use the medium as the control; the combined drug regimen is two antibodies according to 1: The mass ratio of 1 is mixed with the drug, and the dosage of each antibody is the same as that of the antibody single drug;
[0164] 4. After 72 hours, add 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com