A chiral imidazolium-containing pyridine amide compound and its preparation method and application
A technology of imidazole pyridine amide and compound, applied in the field of asymmetric hydroboration reaction, can solve problems such as unreported, difficult P ligands and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
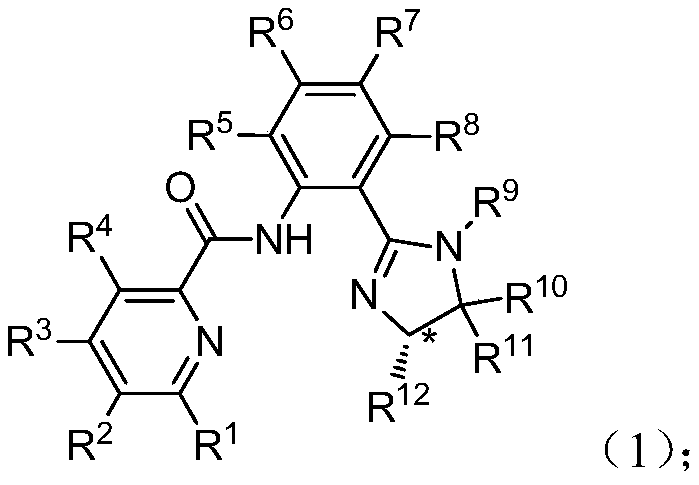
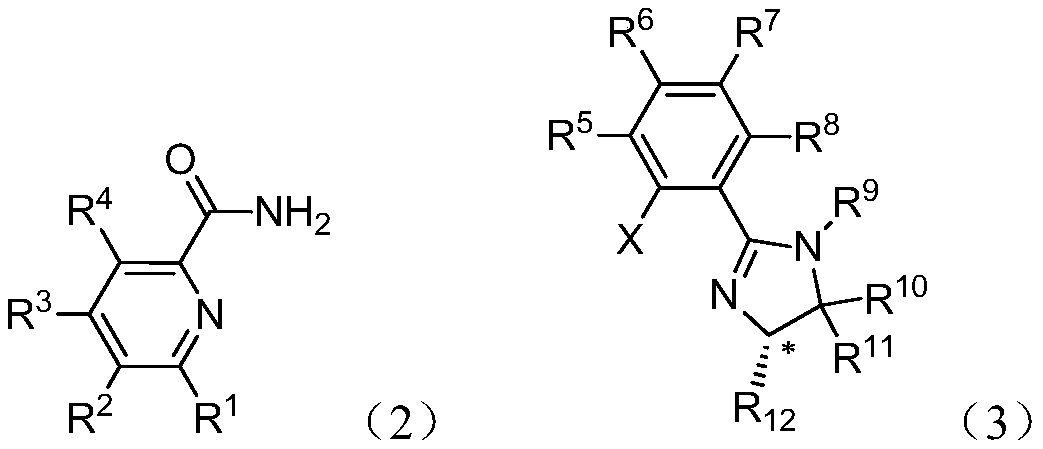
[0063] Example: The formula (2) is commercially available, and the halide formula (3) is prepared according to the literature (Tetrahedron: Asymmetry, 2016, 27, 163-170).
[0064] Preparation of compound formula (1)
example A1
[0065] Example A1: Preparation of Compound A1
[0066]
[0067] Under nitrogen protection, (S)-(1-phenyl-2-(2-iodophenyl)-4-benzyl-4,5-dihydro)-1H-imidazole (0.4438g, 1.01mmol, 1.0equiv) With 2-pyridinecarboxamide (0.1461g, 1.20mmol, 1.2equiv) in 5.0mL dioxane, CuI (0.0191g, 0.1mmol, 10mol%), ethylenediamine (0.0120g, 0.2mmol, 20mol%) , Potassium phosphate trihydrate (0.5326g, 2.0mmol, 2.0equiv), reacted at 60°C for 48 hours, petroleum ether: ethyl acetate = 3:1 through the column, to obtain 0.2509g (0.58mmol, yield 58%) containing imidazopyridine Amide compound A1.
[0068] 1 H NMR(400MHz, CDCl 3 )δ12.79(s,1H), 8.80(d,J=8.4Hz,1H), 8.59(d,J=4.8Hz,1H), 8.31(d,J=8.0Hz,1H), 7.89(td, J=7.6,1.6Hz,1H),7.48-7.34(m,2H),7.36-7.25(m,4H),7.23-7.18(m,1H),7.16-7.02(m,3H),6.97-6.86( m, 2H), 6.68 (d, J = 8.4 Hz 2H), 4.75-4.65 (m, 1H), 4.14 (dd, J = 10.4, 9.6 Hz, 1H), 3.65-3.50 (m, 2H), 2.90 ( dd,J=14.0,9.2Hz,1H); 13 C NMR(101MHz, CDCl 3 )δ163.1, 159.8, 150.6, 148.1, 142.7, 138.5, 137.7, 137.3...
example A2
[0069] Example A2: Preparation of Compound A2
[0070]
[0071] Under nitrogen protection, (S)-(1-phenyl-2-(2-iodophenyl)-4-phenyl-4,5-dihydro)-1H-imidazole (0.4243g, 1.00mmol, 1.0equiv) With 6-methyl-2-pyridinecarboxamide (0.1634g, 1.20mmol, 1.2equiv) in 5.0mL dioxane, CuI (0.0381g, 0.2mmol, 20mol%), ethylenediamine (0.0120g, 0.2 mmol, 20mol%), potassium phosphate (0.4246g, 2.0mmol, 2.0equiv), reacted at 100°C for 24 hours, petroleum ether: ethyl acetate = 3:1 through the column to obtain 0.2361g (0.53mmol, 53%) containing imidazole Compound A2 of pyridine amide.
[0072] 1 H NMR(400MHz, CDCl 3 )δ12.84(s,1H), 8.75(d,J=8.4Hz,1H), 8.06(d,J=7.6Hz,1H), 7.72(t,J=7.6Hz,1H),7.45-7.37( m,3H),7.30-7.16(m,5H),7.13(t,J=8.0Hz,2H),6.99-6.80(m,2H),6.80(dd,J=8.4,1.0Hz,2H),5.59 (dd, J = 10.4, 8.8 Hz, 1H), 4.44 (dd, J = 10.8, 9.6 Hz, 1H), 3.94 (dd, J = 9.2, 8.8 Hz, 1H), 2.21 (s, 3H). 13 C NMR(101MHz, CDCl 3 )δ163.5,160.8,157.3,149.8,143.6,142.8,138.0,137.3,130.9,130.2,128.8,128.5,127.3,126.8,12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com