Method for preparing optical pure L-tertiary leucine from active inclusion body
A technology of tert-leucine and inclusion bodies, applied in the field of bioengineering, to achieve the effects of simple process flow, reduced production cost, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Construction of FDH-LeuDH Bifunctional Enzyme Recombinant Strain
[0044] Overlap extension polymerase chain reaction (OE-PCR) was used to construct the FDH-LeuDH fusion enzyme mediated by different connecting peptides, and the construction of the FDH-R1-LeuDH fusion enzyme gene was taken as an example to describe the construction process. First, design primers based on LeuDH, FDH, connecting peptide sequence and restriction site on pET28a plasmid:
[0045] P1: 5'-GGAATTC CATATG AAAATTGTCCTGGTCCTGT-3' (SEQ ID NO 05), the underline is the NdeI restriction site sequence.
[0046] Connecting peptide primers:
[0047] 5’-GCCTATGGCAAACACGATAAAAAG XXX ATGACATTGGAAATCTTCGA-3', XXX refers to the connecting peptide sequence, see Table 1 for details.
[0048] P3: 5'-ATGACATTGGAAATCTTCGAATAT-3' (SEQ ID NO 06).
[0049] P4: 5'-CCG CTCGAG TTACCGGCGACTAATGATGT-3' (SEQ ID NO 07), the underline is the XhoI restriction site sequence.
[0050] Using FDH and LeuDH genes as templ...
Embodiment 2
[0058] Preparation of Bifunctional Enzyme Active Inclusion Body
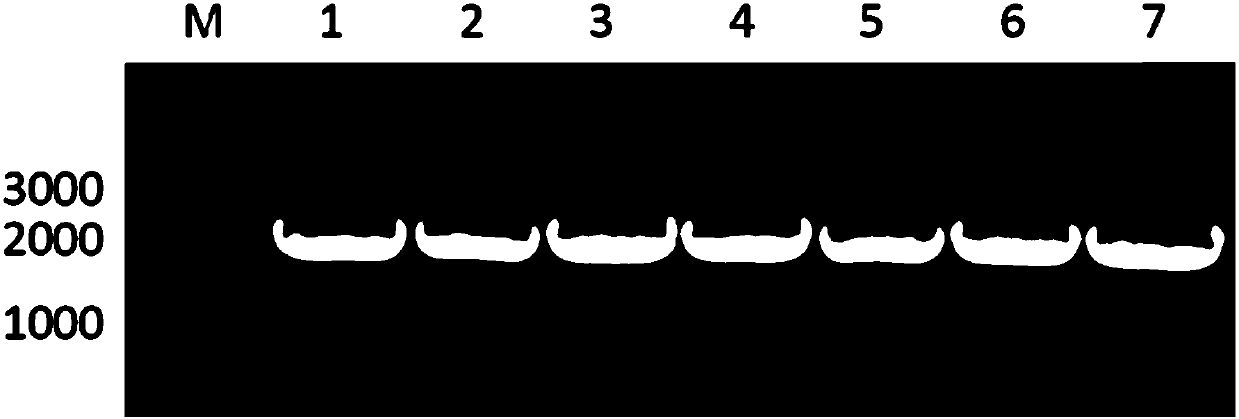
[0059] The bifunctional enzyme recombinant strain was inoculated in LB medium, activated overnight at 37°C, 200rpm, then transferred to Lb medium with an inoculum size of 1%, cultivated at 37°C, 200rpm until the OD600 was about 0.5, and added a final concentration of 0.2 mM IPTG, 16°C, 200rpm induced expression for 24h. After the cultivation was completed, the bacterial cells were collected, washed twice with PBS buffer (pH=7.2), and stored at -80°C until use. Whole-cell SDS-PAGE was used to verify whether the recombinant bifunctional enzyme was successfully expressed, and the results were as follows: figure 2 As shown, where M represents the protein marker, bands 1-9 are FDH single enzyme, LeuDH single enzyme, FDH-DL-LeuDH, FDH-S1-LeuDH, FDH-S2-LeuDH, FDH-S3-LeuDH, FDH-R1 -LeuDH, FDH-R2-LeuDH and FDH-R3-LeuDH, it can be seen that the bifunctional enzymes mediated by the seven connecting peptides were all suc...
Embodiment 3
[0062] Characterization of Bifunctional Enzyme Active Inclusion Body
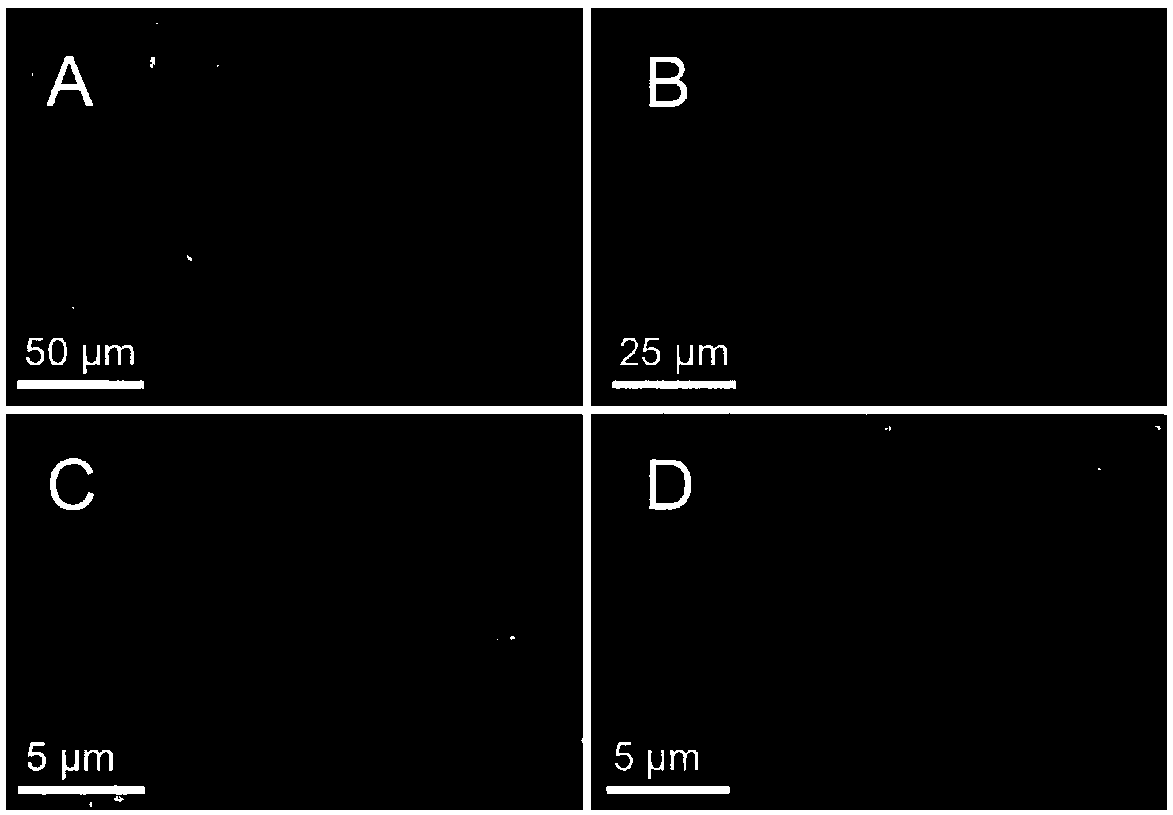
[0063] The morphology of inclusion bodies was directly observed by scanning electron microscopy. The sample preparation method is as follows: 5 μL of the inclusion body sample was dropped on a single crystal silicon wafer, air-dried overnight, and then coated with about 2 nm thick platinum in a JFC-1600 (JEOL, Tokyo, Japan) sputtering apparatus (sputtering condition : 10mA, 30s), and then put the coated sample into a field emission Sigma scanning electron microscope (Carl-Zeiss AG, Germany) for observation. image 3 It is the SEM structure diagram of partial inclusion body, where A is FDH-R1-LeuDH, B is FDH-R2-LeuDH, C is FDH-S1-LeuDH, D is FDH-S2-LeuDH, it can be seen that the rigid linker peptide mediates The inclusion body with bifunctional enzyme activity showed a sheet structure, while the inclusion body with bifunctional enzyme activity mediated by a flexible linker peptide presented an irregular bal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com