Application of astilbin in preparing medicine for treating gastric cancer
A technology for astilbin and gastric cancer, which is applied to the application field of astilbin in the preparation of a drug for the treatment of gastric cancer, can solve the problems of insignificant treatment effect, low treatment compliance, small toxic and side effects, etc., so as to enhance the immune function and effect of the body Good and quick results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
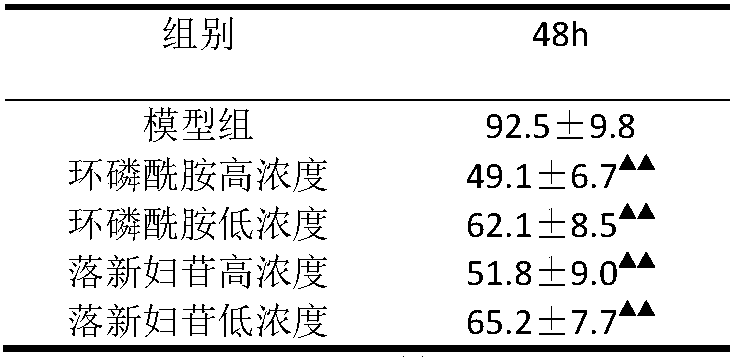
[0020] Astilbin inhibits the proliferation of human gastric cancer cell line MKN-45 in vitro
[0021] Human gastric cancer cell line MKN-45 cells were cultured in a cell culture box with IMDM medium containing 10% fetal bovine serum. When the cells grow well and the walls of the cell culture flask are basically covered, the cells are digested, 100μL of the cell suspension is inoculated into a 96-well plate at a concentration of 1×106 / L, and then divided into a model group and a cyclophosphamide high-concentration group (cyclophosphate Amide 10-6mol / L), cyclophosphamide low concentration group (10-8mol / L), astilbin high concentration group (10-5mol / L), astilbin low concentration group (10-7mol / L), Make 5 holes for each concentration group. After 48 hours of incubation, the cell supernatant was collected, and after centrifugation, the survival rate of MKN-45 cells was detected according to the instructions of the MTT kit.
[0022] The experimental results are shown in Table 1. The...
Embodiment 2
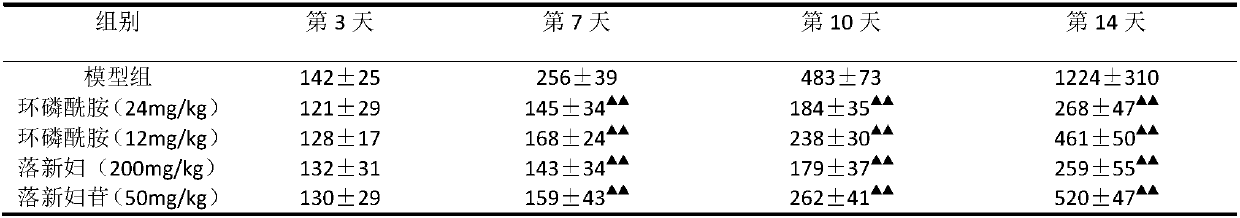
[0027] Astilbin inhibits tumor growth of human gastric cancer cell line MKN-45 in vivo
[0028] Take the human gastric cancer cell line MKN-45 in the logarithmic growth phase, and adjust the cell concentration to 1×10 after washing with PBS 7 / mL, used to inoculate immunodeficient mice. Take 40 BALB / c female nude mice, and inoculate 5×10 tumor cells in the shoulder blades of each mouse 6 Each, wait for the tumor to grow to 100mm 3 Later, they were randomly divided into 5 groups, namely cyclophosphamide high-dose group (24mg / kg), cyclophosphamide low-dose group (12mg / kg), astilbin high-dose group (200mg / kg), astilbin low-dose group Dose group (50mg / kg) and model group. Gavage was given once a day, and the model group was given the same volume of drinking water by gavage for 2 weeks. The tumor volume was measured twice a week, and the mouse body weight was measured at the end of the experiment.
[0029] The experimental results are shown in Table 2 and Table 3. The tumor growth is ...
Embodiment 3
[0037] Zhou, male, 57 years old, suffered from stomach problems for 2 years. His symptoms included upper abdominal discomfort and fullness, loss of appetite, often accompanied by nausea, belching, acid reflux and vomiting, and occasionally hematemesis. He was diagnosed as stomach cancer by the hospital. 2g of astilbin was taken in 2 doses, 4 weeks as a course of treatment. After 3 courses of treatment, the appetite improved, the body became stronger, and the patient's stomach pain, nausea, vomiting, and vomiting were gradually relieved. After 6 consecutive courses of treatment, all symptoms disappeared, the tumor no longer exists, and everything is normal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com