Serum marker for detecting pulmonary embolism and application thereof
A technology of serum markers and pulmonary embolism, applied in the field of medicine and biology, can solve problems such as unclear diagnosis of pulmonary embolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Differential verification of serological markers
[0030] Randomly grouped according to gender and age, 32 healthy people were selected as controls, and 31 patients with aortic aneurysm / aortic dissection were selected. The expression levels of protein factors in the extracted serum samples were detected using the QAH-CUST chip.
[0031] Experimental steps:
[0032] 1. Complete drying of the slide chip: Take the slide chip out of the box, and after equilibrating at room temperature for 20-30 minutes, open the packaging bag, uncover the sealing strip, and then put the chip in a vacuum desiccator or dry at room temperature for 1- 2 hours.
[0033] 2. Configuration of standard products:
[0034] (1) Add 500 μL of sample diluent to the vial of cytokine standard mixture and redissolve the standard. Before opening the vial, give it a quick centrifuge and gently pipette up and down to dissolve the powder. Label this vial as Std 1.
[0035] (2) Mark six clean cent...
Embodiment 2
[0069] Example 2, large sample verification of pulmonary embolism serological markers
[0070] Randomly grouped according to gender and age, 101 healthy people were selected as controls, 234 patients with aortic aneurysm / aortic dissection, and 203 patients with pulmonary embolism. The ELISA experiment of serum protein markers was carried out, and the corresponding RayBiotechHuman ELISA Kit was used to detect the expression levels of the nine protein factors obtained in Example 1, to verify whether the histone factors could effectively distinguish patients with aortic aneurysm / aortic dissection.
[0071] The experimental steps are as follows:
[0072] 1. Reagent preparation
[0073] (1) Equilibrate the kit and samples to room temperature (18-25°C);
[0074] (2) Samples According to the pre-test results, the serum samples of the persons to be tested were diluted according to the multiples shown in Table 2 below.
[0075] Table 2. Dilution multiples when detecting different di...
Embodiment 3
[0110] Embodiment 3, optimal diagnosis combination protein factor analysis
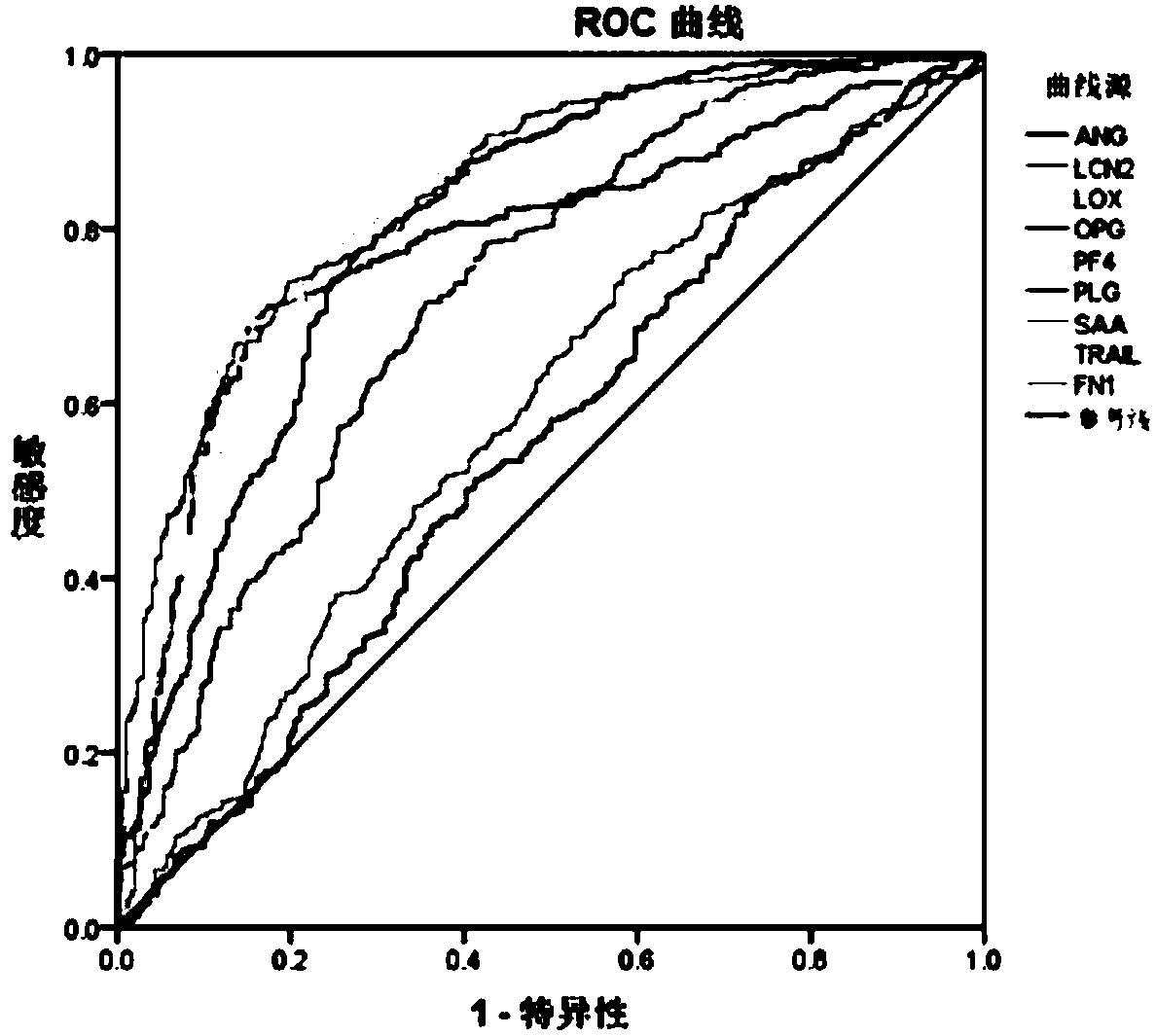
[0111] Through the ROC statistical analysis of the combined effect of each factor, it is determined to distinguish aortic aneurysm / aortic dissection (A) from healthy people (H), pulmonary embolism (PE) from healthy people (H), aortic aneurysm / aortic dissection ( A) Best diagnostic combination with pulmonary embolism (PE).
[0112] Through further analysis, it is determined that the best diagnostic factor for distinguishing between pulmonary embolism (PE) and healthy people (H) is TRAIL, and the serum of 423 routine healthy people and the serum of 298 routine pulmonary embolism patients are selected again, according to embodiment 1-2 Methods Serological marker detection, the results are as follows in Table 6 or Figure 6 shown.
[0113] Table 6, the concentration of the serum marker TRAIL of healthy people and patients with aortic aneurysm / dissection
[0114]
Healthy person (H)
pulm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com