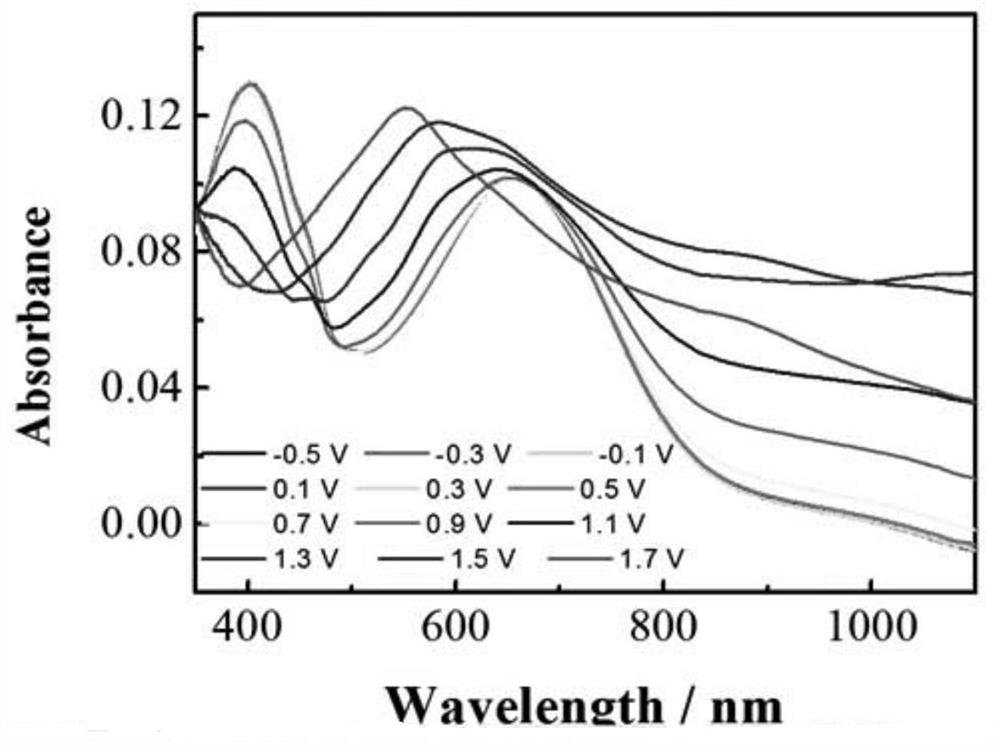
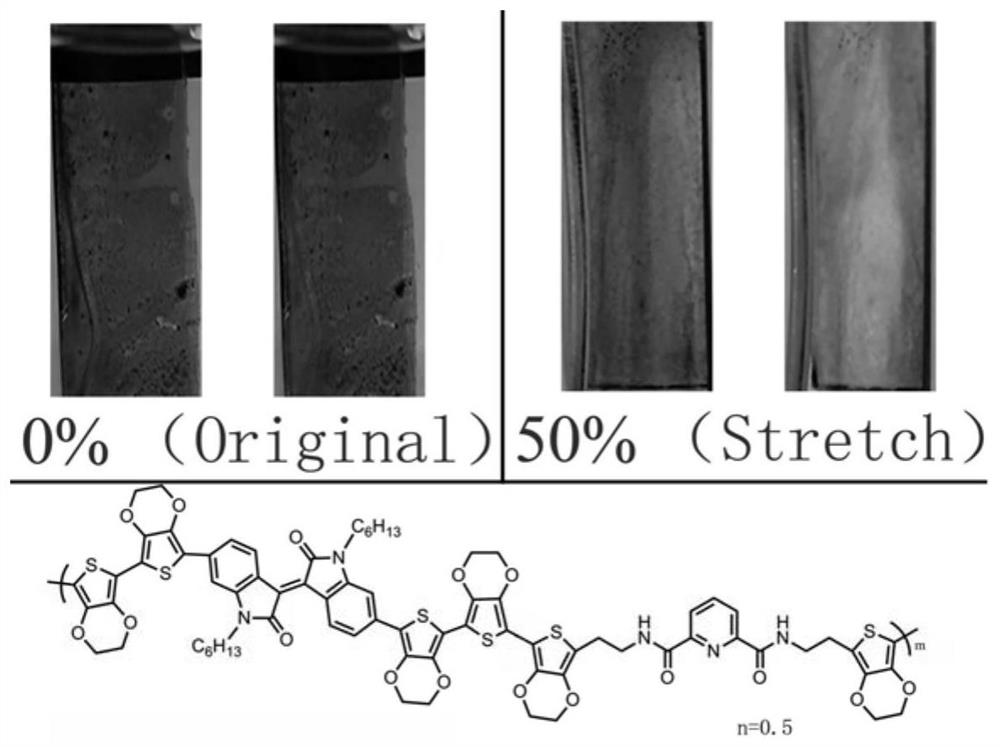
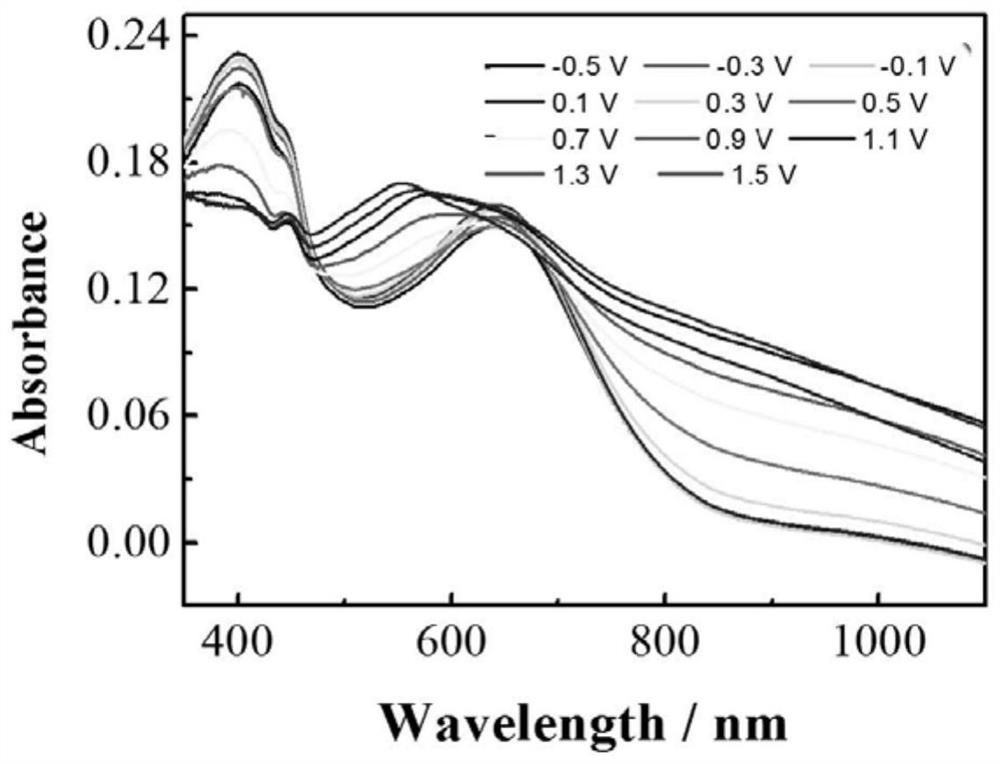
A hydrogen bond crosslinked intrinsically stretchable electrochromic polymer and its preparation method
An electrochromic, polymer technology, applied in the field of electrochromic materials, which can solve the problems of weak force and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A hydrogen-bonded cross-linked intrinsically stretchable electrochromic polymer based on isoindigos, prepared by the following steps:
[0048] (1) Synthesis of stretchable group PDCAs
[0049]
[0050] (1a) Using 2,6-pyridinedicarboxylic acid (10.03g, 0.06mol) as raw material, react with oxalyl chloride (22.85g, 0.18mol) to generate 2,6-pyridinedicarboxylic acid chloride;
[0051] (1b) 2,6-Pyridinedicarboxylic acid chloride (2.98g, 0.18mol) and 2-ethylamine EDOT (6.00g, 0.32mol) in a mixed solution of triethylamine (6mL) and CH2Cl2 (36mL) for one-step synthesis of PDCA short chain,
[0052] (1c) PDCA short chain (1.3g, 3.37mmol) reacts with LDA (3.37mL, 6.74mmol), tributyltin chloride (2.19g, 6.74mmol) to obtain tin-butylated PDCAs, after separation and purification, as Use later.
[0053] (2) Synthesis of donor group BisEDOT
[0054]
[0055] (2a) With EDOT (7.50g, 50mmol) as raw material, THF (50mL) as solvent, react with n-butyllithium (53mL, 85.00mmol) at ...
Embodiment 2
[0068] A hydrogen-bonded cross-linked intrinsically stretchable electrochromic polymer based on benzotriazoles is prepared through the following steps:
[0069] (1) Synthesis of stretchable group PDCAs
[0070]
[0071] (1a) Using 2,6-pyridinedicarboxylic acid (10.03g, 0.06mol) as raw material, react with oxalyl chloride (22.85g, 0.18mol) to generate 2,6-pyridinedicarboxylic acid chloride;
[0072] (1b) 2,6-Pyridinedicarboxylic acid chloride (2.98g, 0.18mol) and 2-ethylamine EDOT (6.00g, 0.32mol) in a mixed solution of triethylamine (6mL) and CH2Cl2 (36mL) for one-step synthesis of PDCA short chain,
[0073] (1c) PDCA short chain (1.3g, 3.37mmol) reacts with LDA (3.37mL, 6.74mmol), tributyltin chloride (2.19g, 6.74mmol) to obtain tin-butylated PDCAs, after separation and purification, as Use later.
[0074] (2) Synthesis of donor group BisEDOT
[0075]
[0076] (2a) With EDOT (7.50g, 50mmol) as raw material, THF (50mL) as solvent, react with n-butyllithium (53mL, 85.00...
Embodiment 3
[0090] A hydrogen-bonded cross-linked intrinsically stretchable electrochromic polymer based on PEDOT oligomers, prepared by the following steps:
[0091] (1) Synthesis of stretchable group PDCAs
[0092]
[0093] (1a) Using 2,6-pyridinedicarboxylic acid (10.03g, 0.06mol) as raw material, react with oxalyl chloride (22.85g, 0.18mol) to generate 2,6-pyridinedicarboxylic acid chloride;
[0094] (1b) 2,6-Pyridinedicarboxylic acid chloride (2.98g, 0.18mol) and 2-ethylamine EDOT (6.00g, 0.32mol) in a mixed solution of triethylamine (6mL) and CH2Cl2 (36mL) for one-step synthesis of PDCA short chain,
[0095] (1c) PDCA short chain (1.3g, 3.37mmol) reacts with LDA (3.37mL, 6.74mmol), tributyltin chloride (2.19g, 6.74mmol) to obtain tin-butylated PDCAs, after separation and purification, as Use later.
[0096] (2) Synthesis of donor group BisEDOT
[0097]
[0098] (2a) With EDOT (7.50g, 50mmol) as raw material, THF (50mL) as solvent, react with n-butyllithium (53mL, 85.00mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com