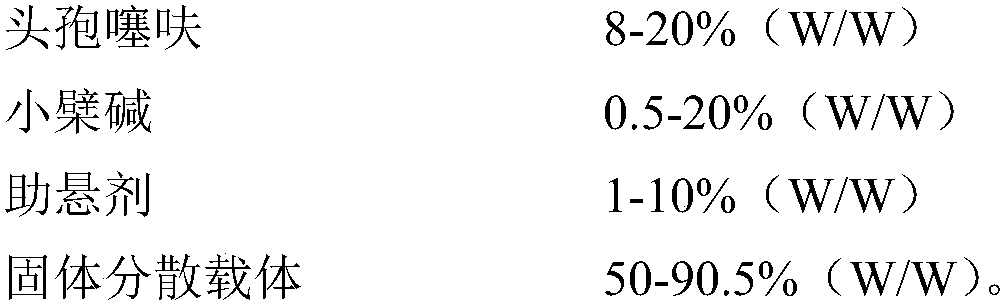Dry-period compound ceftiofur breast injectant composition, and preparation method thereof
A technology of breast injection and ceftiofur, which is applied in the formulation and preparation of compound ceftiofur breast injection during the dry milk period, can solve the problem of high transportation and storage conditions, unprotected milk cow teats, and clinical curative effect of injection Worrying and other problems, to achieve good clinical effect, stable and feasible formulation process, good drug absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0033] Embodiment 7: curative effect test
[0034] 1. Materials
[0035] Blank control group: normal saline
[0036] Drug control group 1: 10% ceftiofur hydrochloride injection, commercially available.
[0037] Drug control group 2: a sample prepared by the method of Example 6 of a patented ceftiofur hydrochloride breast injection (dry milk period) (105726461A, publication date: 2016-07-06)
[0038] Test sample group: (Example 2) Compound ceftiofur breast injection (dry period).
[0039] 2. Method
[0040] Select 32 healthy dairy cows that are about to stop milking, and divide them into 4 groups, 8 in each group; after the last time to squeeze out the residual milk in the udder, the blank control group received normal saline, and the drug control group 1 received 10% ceftiofur hydrochloride. Injection, drug control group 2 prepares samples (ceftiofur hydrochloride 6kg, stearic acid 3kg, glyceryl stearate 3kg, Tween 801.5kg, Span 20 1.5kg, coconut oil 45kg) with certain pat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com