Preparation method of bepotastine besilate pharmaceutical composition
A technology of bepotastine bepotastine and its composition, which is applied in the field of preparation of bepotastine bepotastine pharmaceutical composition, can solve the problems of complex operation, high production line cost, and large quality difference, and achieve uniform dispersion and prolonged solidification Good time and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
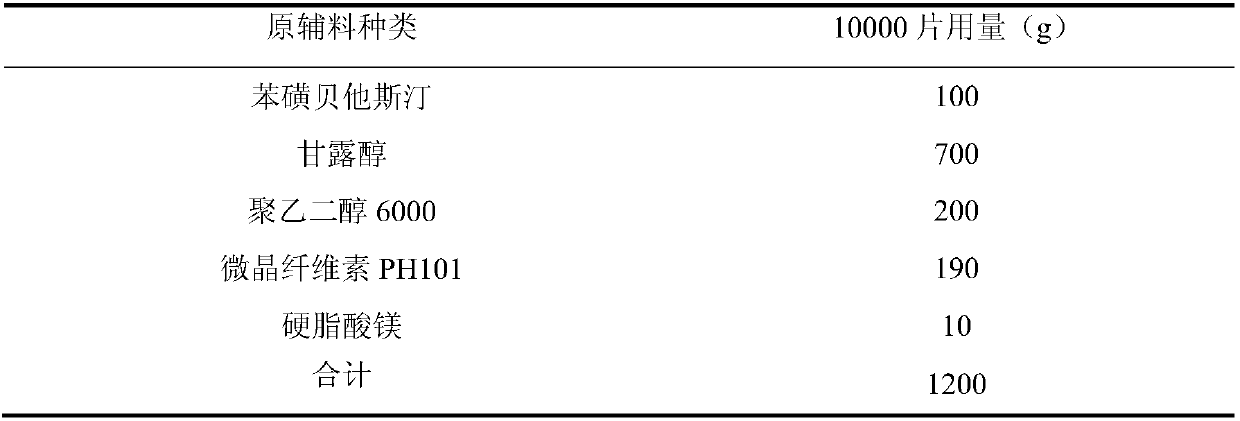
[0028] Example 1: The mass ratio of polyethylene glycol 6000 to water is 2:1
[0029]
[0030] Preparation Process:
[0031] 1) Pretreatment: Mechanically pulverize the raw materials through an 80-mesh sieve for subsequent use; pass the auxiliary materials through an 80-mesh sieve for subsequent use;
[0032] 2) Adhesive preparation: Prescribed amount of polyethylene glycol 6000 and appropriate amount of water were placed in a water bath and heated at 85°C;
[0033] 3) Granulation steps: 1. Put the raw material drug and corresponding auxiliary materials in a wet granulator and mix them evenly; 2. Disperse the melted polyethylene glycol 6000 and water evenly according to the ratio of 2:1. (60s) Add to the wet granulator to make PEG6000 evenly dispersed; 3. Add 50g of water to prepare soft material, stir and shear at low speed (60s); granulate, dry, and granulate;
[0034] 4) Blending step: specifically, uniformly mixing the granules obtained in step 3) with additional auxi...
Embodiment 2
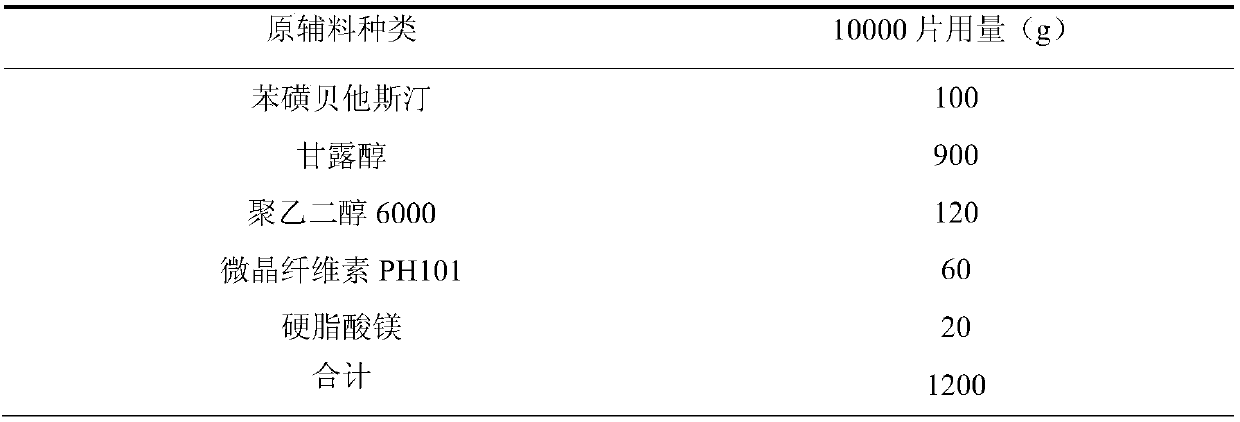
[0036] Example 2: The mass ratio of polyethylene glycol 6000 to water is 20:1
[0037]
[0038] Preparation Process:
[0039] 1) Pretreatment: Mechanically pulverize the raw materials through an 80-mesh sieve for subsequent use; pass the auxiliary materials through an 80-mesh sieve for subsequent use;
[0040] 2) Adhesive preparation: Prescribed amount of polyethylene glycol 6000 and appropriate amount of water were placed in a water bath and heated at 70°C;
[0041] 3) Granulation steps: 1. Put the raw material drug and corresponding auxiliary materials in a wet granulator and mix them evenly; 2. Disperse the melted polyethylene glycol 6000 and water according to the ratio of 20:1. (60s) into the wet granulator, so that polyethylene glycol 6000 is evenly dispersed; 3. Add 20g of water to prepare soft materials, stir and shear at low speed (30s); granulate, dry, and granulate;
[0042] 4) Blending step: specifically, uniformly mixing the granules obtained in step 3) with ...
Embodiment 3
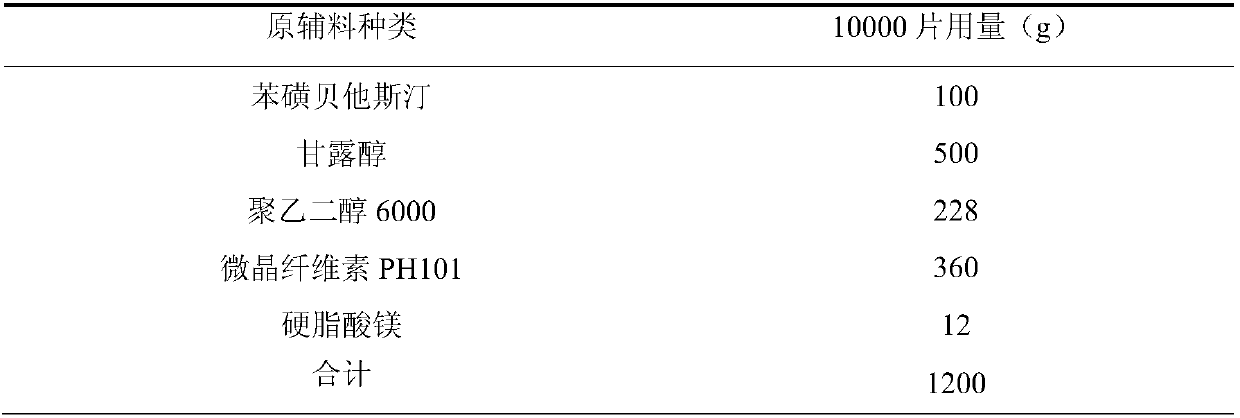
[0044] Example 3: The mass ratio of polyethylene glycol 6000 to water is 5:1
[0045]
[0046] Preparation Process:
[0047] 1) Pretreatment: Mechanically pulverize the raw materials through an 80-mesh sieve for subsequent use; pass the auxiliary materials through an 80-mesh sieve for subsequent use;
[0048] 2) Adhesive preparation: put the prescribed amount of PEG6000 and appropriate amount of water in a water bath and heat at 80°C;
[0049] 3) Granulation steps: 1. Mix the raw materials and corresponding excipients in a wet granulator; 2. Disperse the melted PEG6000 and water at a ratio of 5:1, and add In the wet granulator, PEG6000 is dispersed evenly; 3. Add 10g of water to prepare soft material, stir and shear at low speed (20s); granulate, dry, and granulate;
[0050] 4) Blending step: specifically, uniformly mixing the granules obtained in step 3) with additional auxiliary materials to obtain blended granules;
[0051] 5) Tablet compression and coating, specifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com