Preparation and application of ultra-sensitive high-selectivity hypochlorous acid fluorescence probe
A preparation and selected technology are applied in the field of fluorescein compounds as hypochlorous acid fluorescent probes, which can solve the problems of complex synthesis, poor selectivity and low sensitivity, and achieve the effects of simple synthesis, good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] (Scheme 1) Dissolve 332.3mg (1mmol) fluorescein and 494.4mg (4mmol) dimethylaminothioformyl chloride in dichloromethane and add 460.8mg (4mmol) diisopropylethylamine for normal temperature (25°C) After stirring for 20 h, column chromatography was performed using a dichloromethane system to obtain 304 mg of a yellow solid with a yield of 60%.
[0037] (Scheme 2) Dissolve 332.3mg (1mmol) fluorescein and 741.6mg (6mmol) dimethylaminothioformyl chloride in dichloromethane and add 691.2mg (6mmol) diisopropylethylamine for normal temperature (25°C) Stir for 20 h, and use dichloromethane system for column chromatography to obtain 350 mg of yellow solid with a yield of 69%.
[0038] (Scheme 3) Dissolve 332.3mg (1mmol) fluorescein and 988.8mg (8mmol) dimethylaminothioformyl chloride in dichloromethane and add 921.6mg (8mmol) diisopropylethylamine for normal temperature (25°C) After stirring for 20 h, column chromatography was performed using a dichloromethane system...
Embodiment 2
[0043] The inventors of the present invention have carried out following test:
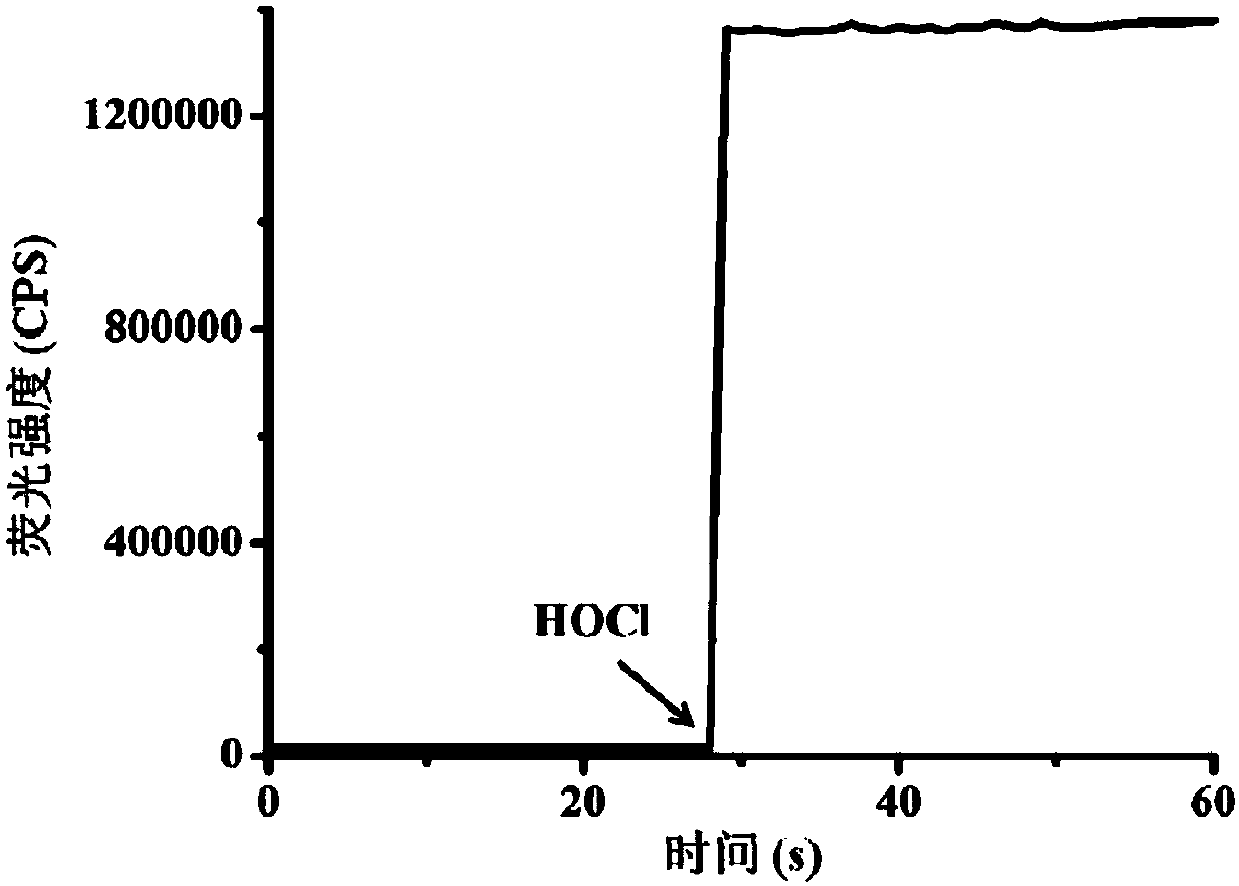
[0044] Test results of probe (5 μM) response time to HOCl (8 μM). The above determinations were carried out in 5mM PBS, pH=7.4 aqueous solution, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25°C. See results figure 1 .
[0045] from figure 1 It can be seen that after adding 8 μM hypochlorous acid, the fluorescence intensity suddenly increases, and the response can be completed within 3s.
Embodiment 3
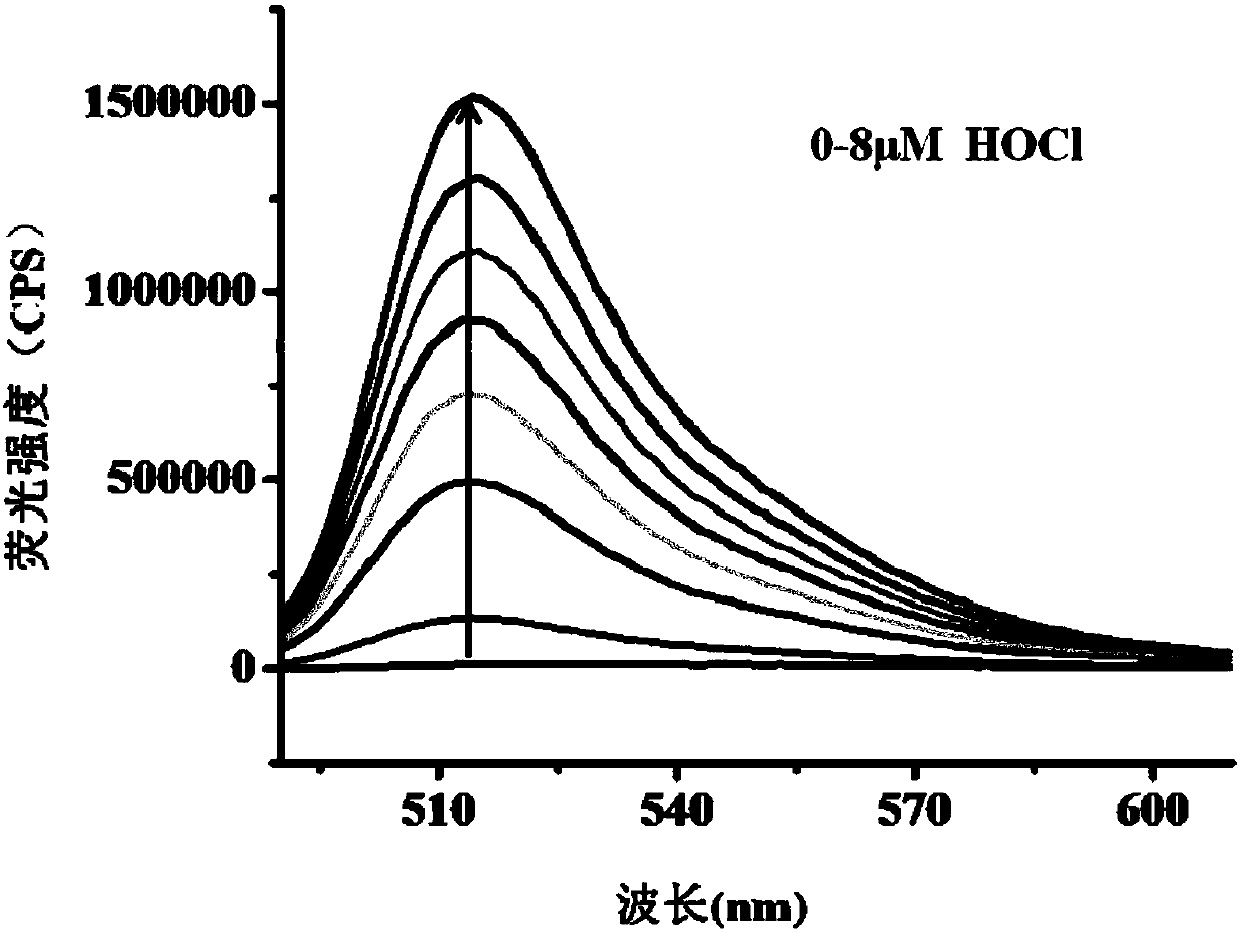
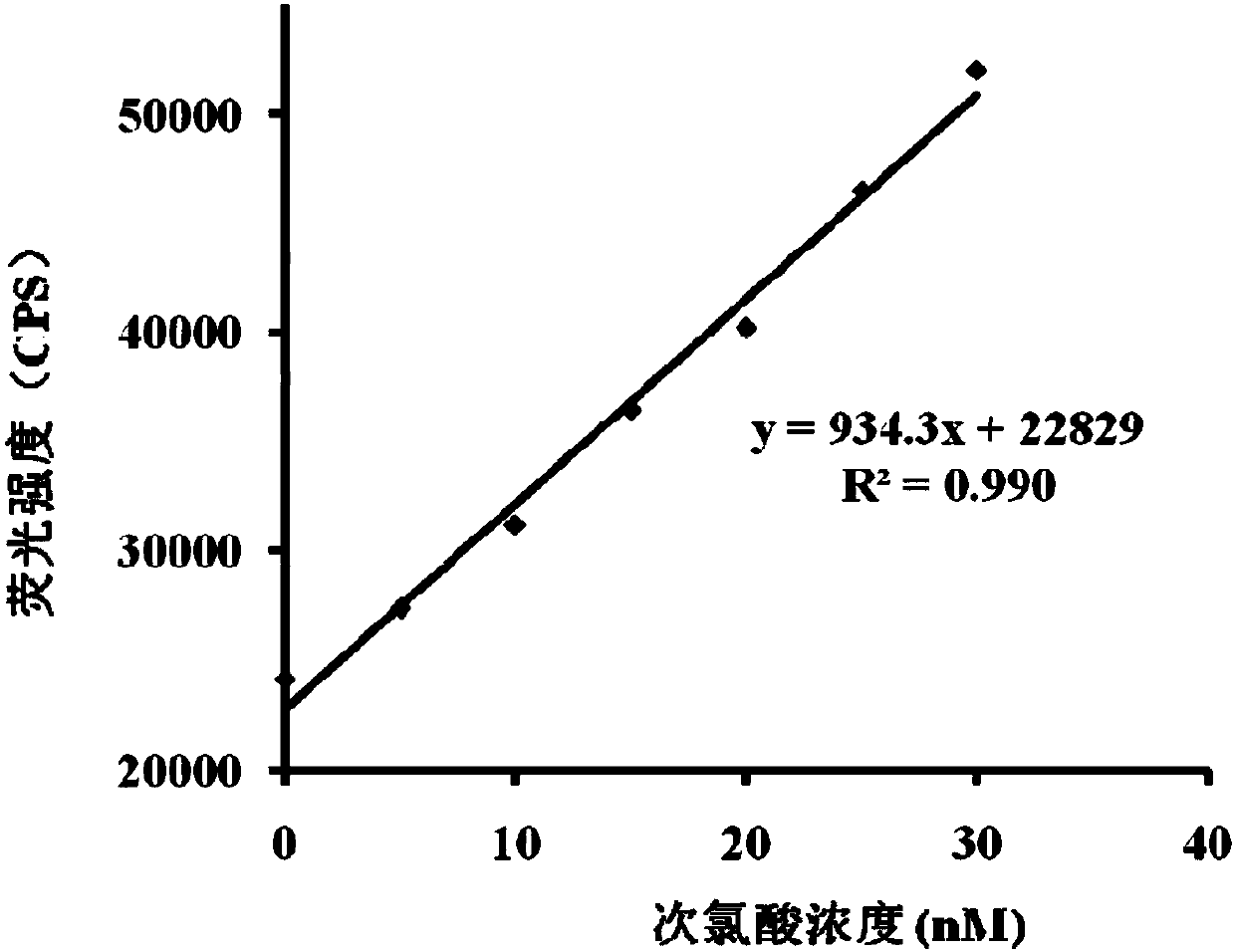
[0047] The effect of different concentrations of hypochlorous acid (0~8μM) on the fluorescence spectrum of the probe (5μM). The above measurements were carried out in an aqueous solution of 5 mM PBS, pH=7.4, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25° C. for 1 min. See results figure 2 and 3 .
[0048] from figure 2 and 3 It can be seen that with the increase of the concentration of hypochlorous acid in the probe solution, the fluorescence spectrum is gradually enhanced, and within the concentration range of hypochlorous acid from 0 to 40 nM, the concentration of hypochlorous acid and the fluorescence intensity show a good linear relationship, and The detection limit was as low as 0.65nM. Therefore, the probe of the present invention can more accurately determine the content of hypochlorous acid in the sample to be tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com