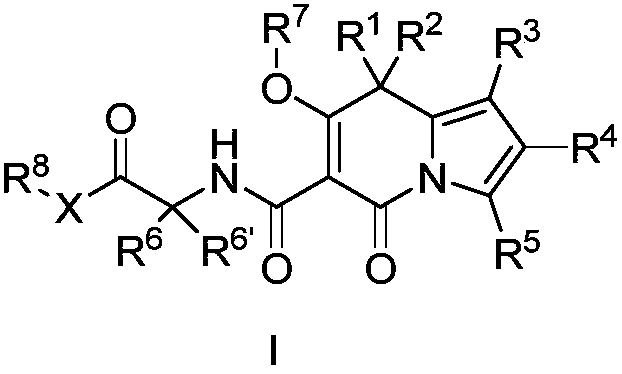
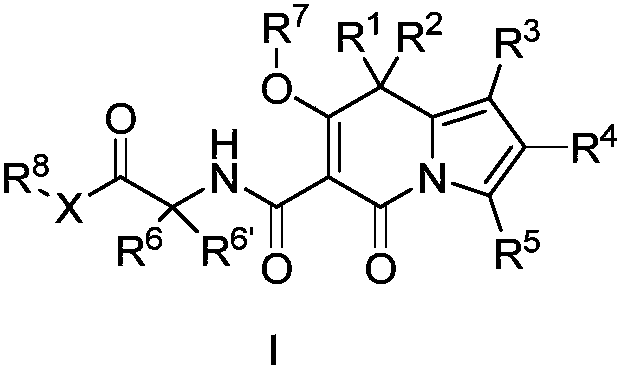
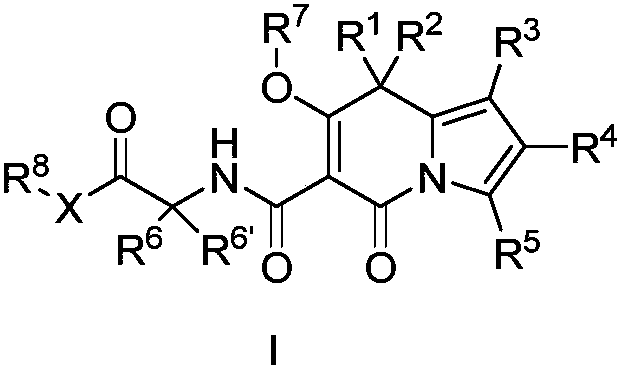
Indolizine derivatives and application thereof in medicines
A compound, isomer mixture technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] (7'-Hydroxy-5'-oxo-1'-phenyl-5'H-spiro[cyclopentane-1,8'-indolezine]-6'-carbonyl)glycine ( 1 )
[0184]
[0185] first step
[0186] 5-(1,3-dioxolan-2-yl)-3-oxo-4-phenylpentanoic acid ethyl ester ( 1b )
[0187]
[0188] Ethyl 3-oxo-4-phenylbutyrate (1.03 g, prepared according to the method described in the literature [European Journal of Medicinal Chemistry, 2014, vol 84, 312-334]) was dissolved in anhydrous tetrahydrofuran (20 ml), and ice Cool in a water bath, slowly add 60% pure mineral oil and sodium hydride mixed solids (200 mg) in small portions under nitrogen flow, and stir for 0.5 hours; then add hexamethylphosphoramide (2.7 g) and 1.6M A solution of n-butyllithium in hexane (3.13 mL); then 2-bromomethyl-1,3-dioxolane (840 mg) was added. The reaction was stirred overnight at room temperature. The reaction solution was carefully poured into saturated ammonium chloride aqueous solution, and then extracted with ethyl acetate; the ethyl acetate layer was...
Embodiment 2
[0212] (1'-(4-fluorophenyl)-7'-hydroxy-5'-oxo-5'H-spiro[cyclopentane-1,8'-indolezine]-6'-carbonyl) Glycine ( 2 )
[0213]
[0214] first step
[0215] 5-(1,3-dioxolan-2-yl)-4-(4-fluorophenyl)-3-oxopentanoic acid ethyl ester ( 2b )
[0216]
[0217] compound 2b According to the first step of embodiment 1 1b The synthetic method preparation, wherein raw material 2a : Ethyl 4-(4-fluorophenyl)-3-oxobutanoate was prepared according to the method described in the literature [European Journal of Medicinal Chemistry, 2014, vol 84, 312-334]. LCMS ESI(+):311(M+1) + .
[0218] second step
[0219] 1-(3-(1,3-dioxolan-2-yl)-2-(4-fluorophenyl)propionyl)cyclopentane-1-carboxylic acid ethyl ester ( 2c )
[0220]
[0221] compound 2c According to the second step of Example 1 1c Synthetic method from the compound 2b preparation. LCMS ESI(+):365(M+1) + .
[0222] third step
[0223] 1-(3-(4-fluorophenyl)-1H-pyrrol 0-2-yl)cyclopentane-1-carboxylic acid ethyl ester ( 2d...
Embodiment 3
[0239] (1'-(4-fluorophenyl)-7'-hydroxy-5'-oxo-5'H-spiro[cyclopentane-1,8'-indolezine]-6'-carbonyl) Ethyl glycinate ( 3 )
[0240]
[0241] compound 2 (1 equivalent) was suspended in ethanol (5 volumes), and thionyl chloride (4 equivalents) was carefully added dropwise; then refluxed until the reaction was complete. Naturally cooled to room temperature and then cooled in an ice-water bath, the solid was collected by filtration and washed with cold ethanol to obtain the compound 3 . LCMS ESI(+):427(M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com