Application of pharmaceutical adjuvant, sophorolipid, in field of medicine
A technology of pharmaceutical excipients and sophorolipids, which is applied in the fields of pharmaceutical formulations, drug delivery, antineoplastic drugs, etc., can solve the problems of unfavorable mass production and batch-to-batch differences, and achieve non-toxic and harmless sources, simple sources, and drug-loading rate-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of sophorolipid
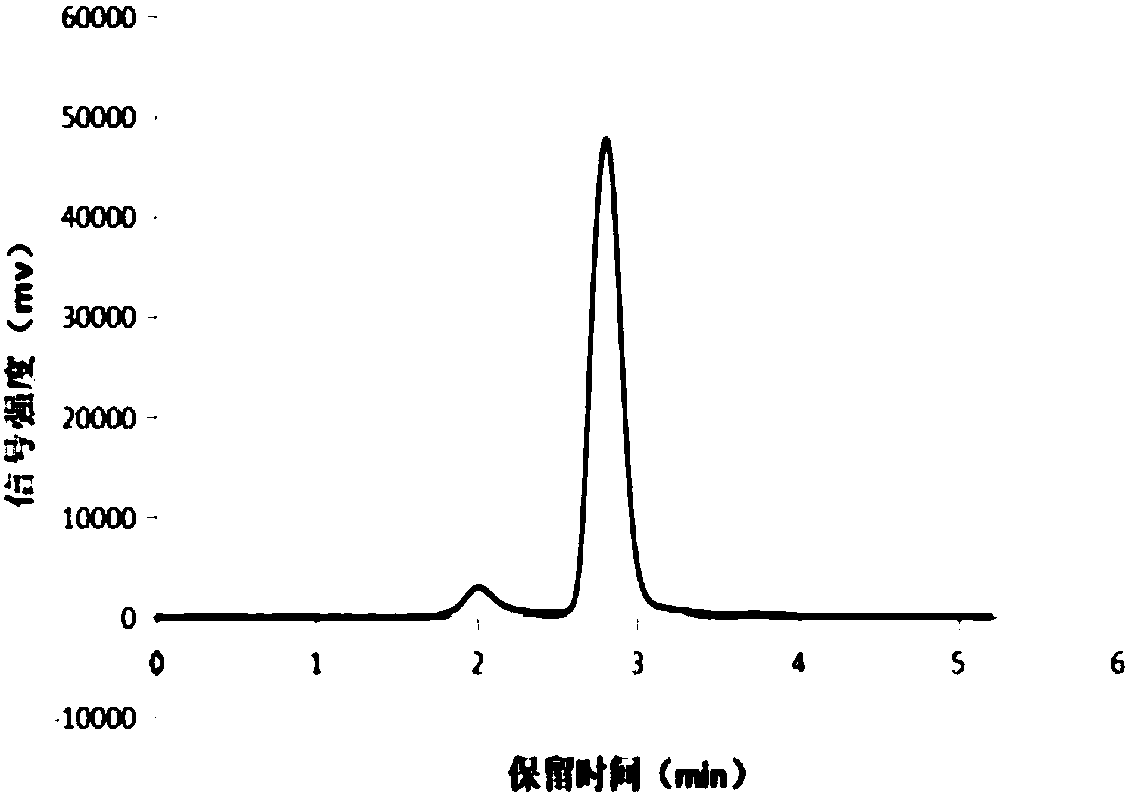
[0043] (1) ferment and cultivate the sophorolipid-producing yeast for 5 days at 28-30°C and 220rpm, wherein the fermentation medium consists of: 6% glucose, 0.3% yeast powder, 0.1% sodium dihydrogen phosphate, dodeca water and Disodium hydrogen phosphate 0.1%, magnesium sulfate heptahydrate 0.05%, oleic acid 6%; (2) the fermentation liquid was extracted with ethyl acetate with a volume ratio of 1:2, and the upper layer extract was distilled under reduced pressure at 50°C, and then mixed with n-hexane Wash 2 times, then distill under reduced pressure at 50°C, and dry in vacuum at 45°C for 24 hours to obtain lactone-type sophorolipids, which is a mixture of lactone-type sophorolipids; 1:2 ethanol was vortex mixed, centrifuged at 6000rpm for 15min, and the supernatant was collected, distilled under reduced pressure at 50°C, washed twice with n-hexane, then distilled under reduced pressure at 50°C, dried under vacuum at 45°C f...
Embodiment 2
[0044] Embodiment 2: positive control group
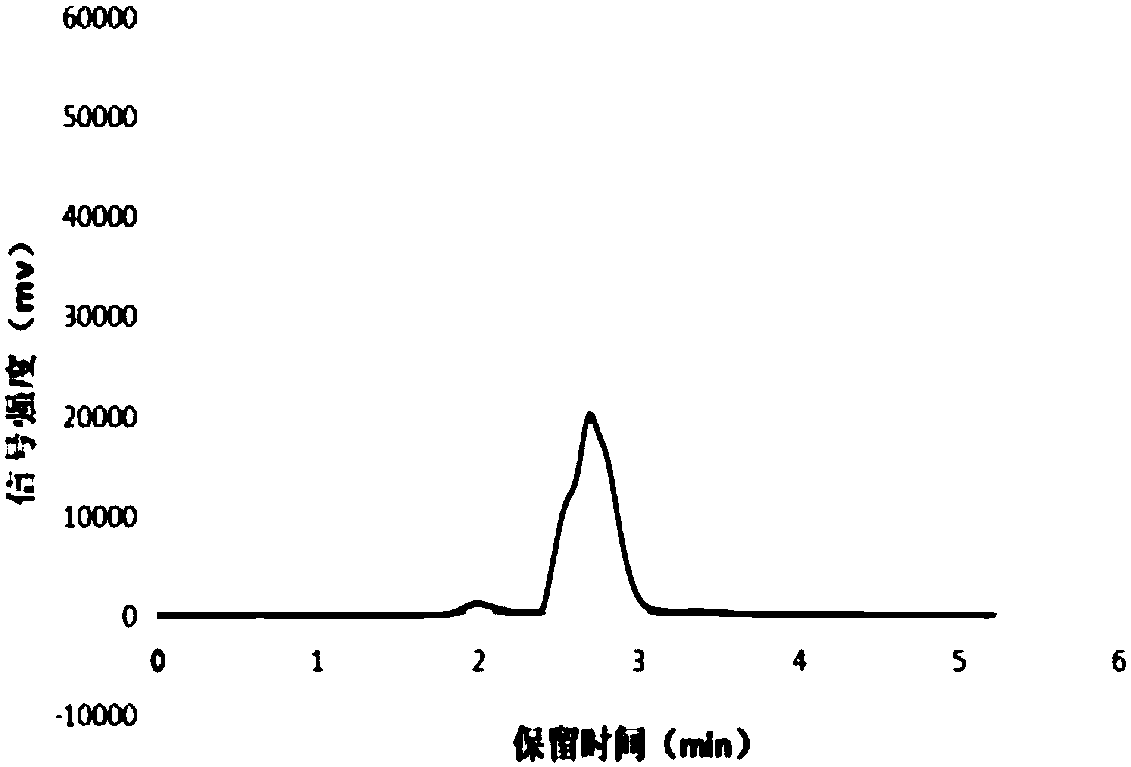
[0045] (1) Weigh 0.1% medicinal Tween and 2.5% glycerol for injection and disperse them in an appropriate amount of water for injection, heat to 70°C, stir until completely dissolved, adjust the pH of the aqueous phase to 5, and set aside; (2) Weigh Etopol Glycoside 0.1% and soybean lecithin 1.8%, add in the mixed solution of long-chain fatty acid triglyceride (LCT) and medium-chain fatty acid triglyceride (MCT), heat to 80 ℃, stir until fully dissolved, set aside; (3) Add the materials in the above (2) to (1), stir until the two phases are mixed evenly, and present a milky white homogeneous phase, dilute with water for injection to a 50ml volumetric flask, and set aside; (4) mix the materials in the above (3) Place in a high-pressure homogenizer, adjust the pressure to 800 bar, homogenize 6 times, and then sterilize under high pressure for 15 minutes to obtain the pharmaceutical excipient.
Embodiment 3
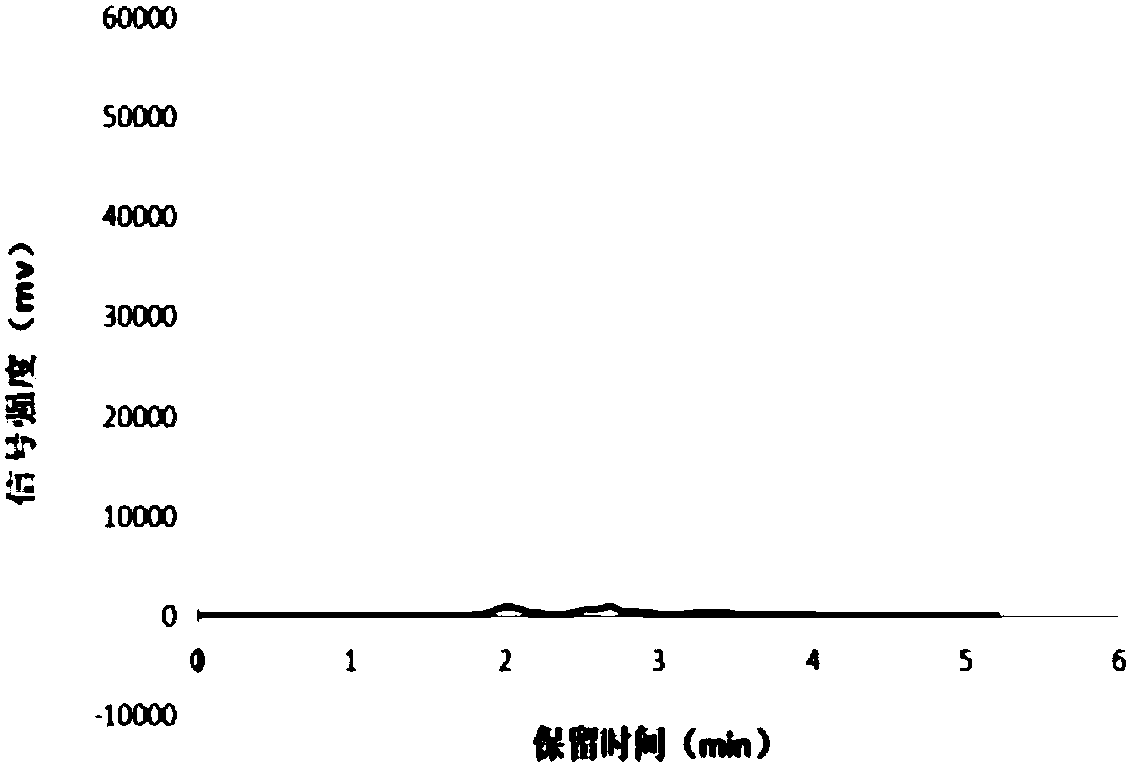
[0046] Example 3, as a blank control, is basically the same as Example 2 but with the following changes: changing medicinal Tween to not adding any emulsifier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com