A method for hydrothermally synthesizing lithium-ion battery cathode materials using siderite
A lithium-ion battery and positive electrode material technology, applied in the field of electrochemistry, can solve the problems of high risk factor, difficult operation, impurity elements, and long process, and achieve the effects of high safety factor, improved electrical conductivity, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one is example with the siderite that contains 1mol iron carbonate, and the concentration of phosphoric acid is 3mol / L, and concrete preparation method is:
[0030] Step 1: Dissolving Siderite
[0031] According to the molar ratio of iron to pure phosphoric acid is 2:4, directly add H 3 PO is calculated as 2 mol of phosphoric acid, the reaction temperature is 70°C, and the reaction time is 2 hours. After fully reacting, filter to obtain the iron solution. The chemical reaction formula is:
[0032] FeCO 3 +2H 3 PO 4 =Fe 2+ +2(H 2 PO 4 ) - +H 2 O+CO 2
[0033] The second step: the solution of iron and lithium hydroxide hydrothermal reaction
[0034] According to the molar ratio of iron to lithium hydroxide is 1:4, 4moL lithium hydroxide is directly added to the iron solution, and lithium iron phosphate is precipitated after fully reacting under hydrothermal conditions, and then filtered to obtain iron phosphate, the positive electrode material of lit...
Embodiment 2
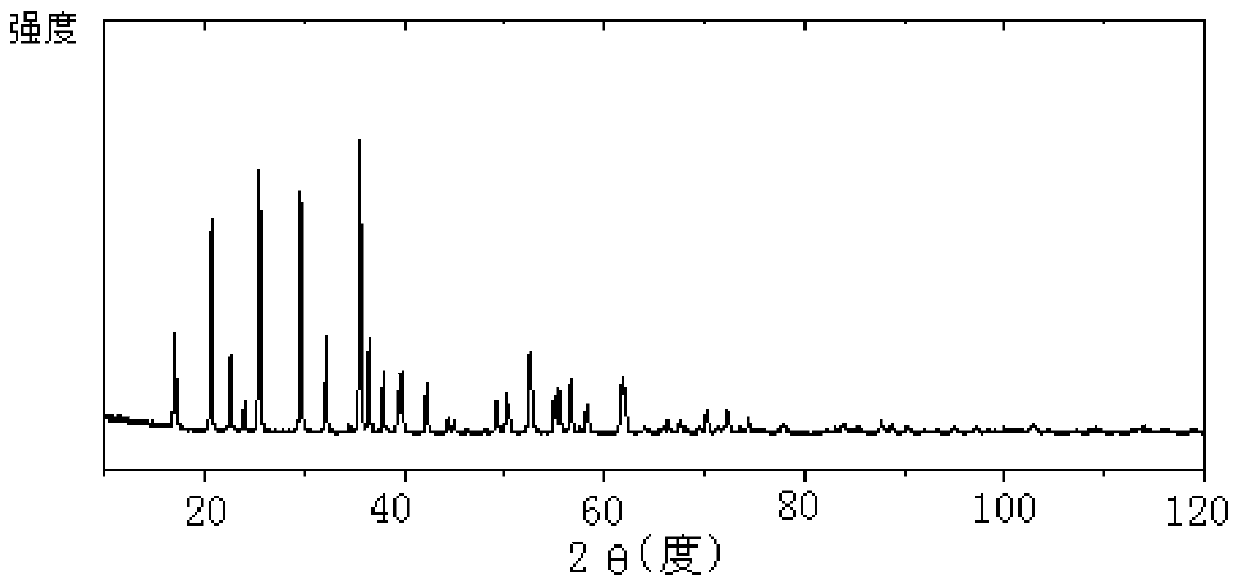
[0036] The scanning electron microscope and X-ray powder diffraction pattern of the lithium iron phosphate prepared in embodiment two are shown in Figure 1-2 .
[0037]With the lithium iron phosphate prepared in Example 2 as the positive electrode and the graphite as the negative electrode, the battery voltage can be made into 3V, and the capacity of the 18650 battery is 1300mA h lithium-ion battery.
[0038] Embodiment two
[0039] Embodiment two is example with the siderite that contains 1mol iron carbonate, and the concentration of phosphoric acid is 0.2mol / L, and concrete preparation method is:
[0040] Step 1: Dissolving Siderite
[0041] According to the molar ratio of iron and phosphorus is 2:8, directly add H 3 PO4 is calculated as 4mol phosphoric acid, the reaction temperature is 50°C, and the reaction time is 5h. After fully reacting, filter to obtain the iron solution. The chemical reaction formula is:
[0042] FeCO 3 +2H 3 PO 4 =Fe 2+ +2(H 2 PO 4 ) - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com