Organic porous material with graphite phase C3N4 structure as well as preparation and application of organic porous material
A porous material, C3N4 technology, applied in water/sludge/sewage treatment, organic compound/hydride/coordination complex catalyst, hydrogen production, etc., can solve the problems of low specific surface area and single energy band structure, etc. Achieve the effect of increased specific surface area, simple process and rapid degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
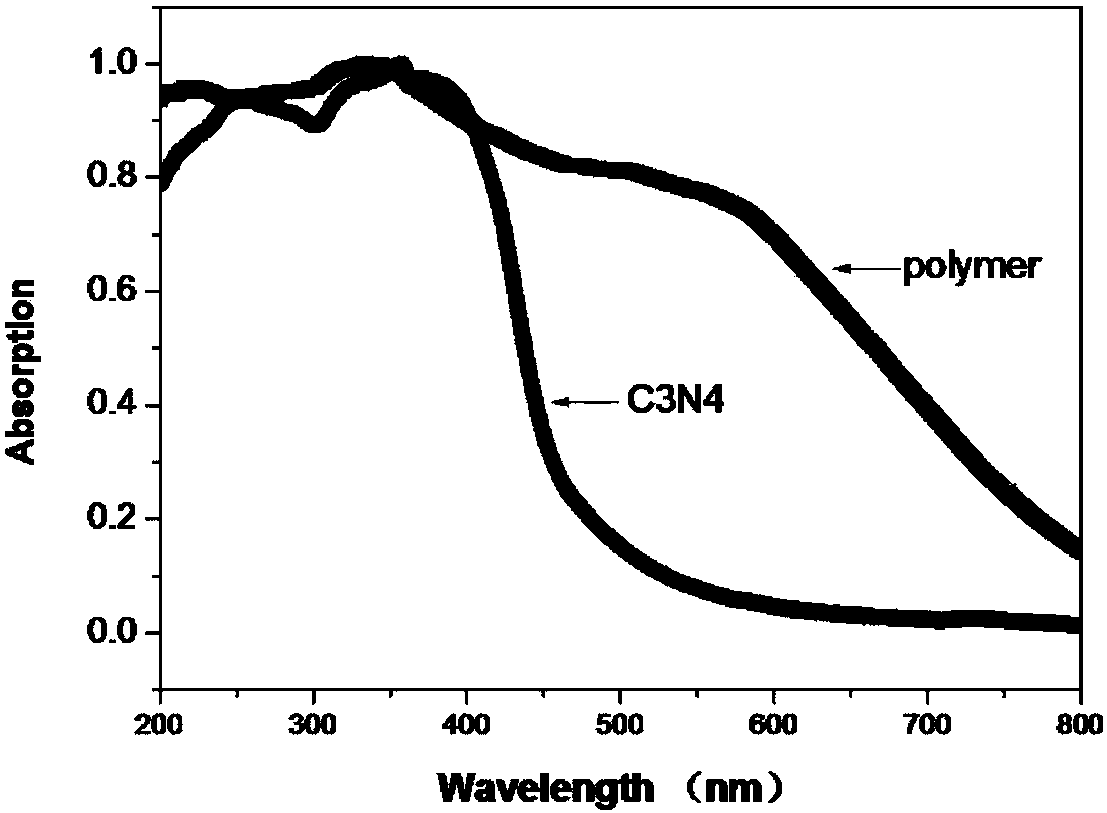
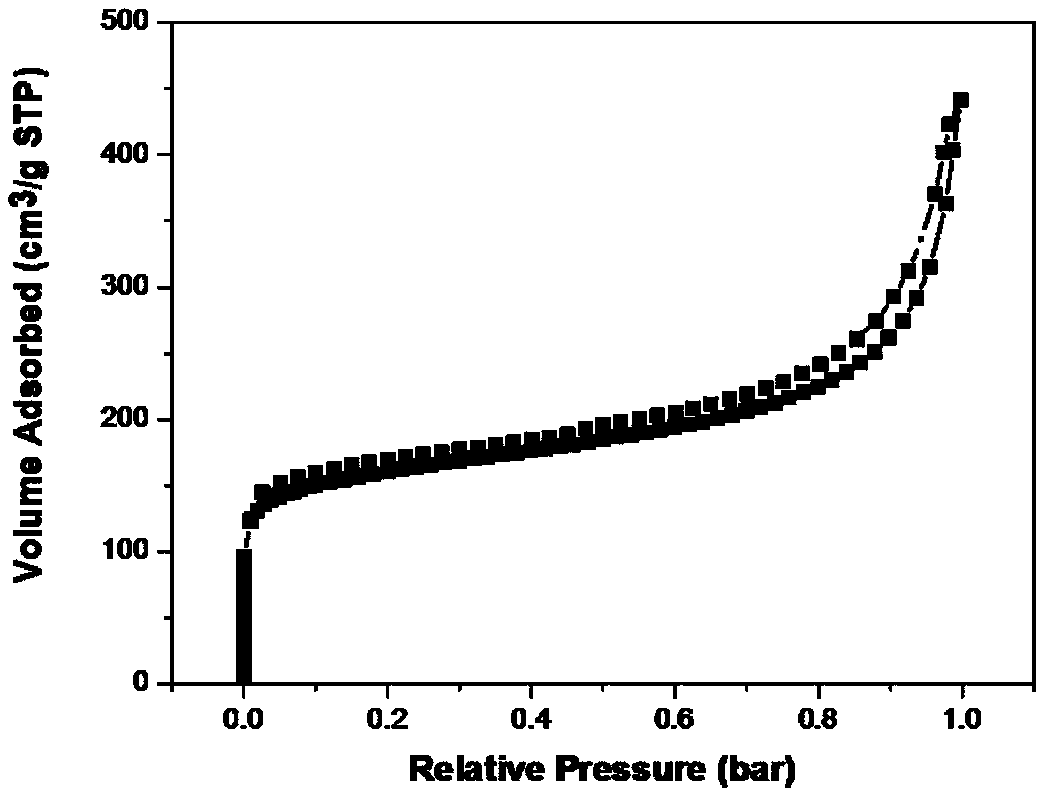
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of the above-mentioned organic porous material comprises the following steps: using 1,3,5-trialdehyde phloroglucinol (Tp) as an aldehyde group building unit, and using triamino-three-triazolidine ring (melem) as an amino building block The unit is obtained by adding an organic solvent and undergoing a Schiff base reaction and then purifying by methanol or tetrahydrofuran by Soxhlet. The molar ratio of aldehyde group to amino group is 2:0.8~2.2; the ratio of aldehyde group to amino group is too high or too low, resulting in the inability to form complete pores, the reaction temperature is 80~150°C; the reaction time is 12~48h.
[0050] Wherein the amino structural unit may also include one or more dibasic or polybasic amino compounds. The dibasic or polybasic amino compound is an aromatic ring, heterocyclic or condensed ring compound of dibasic or polybasic amino groups. The dibasic or polyaminoaromatic compound is preferably tris(4-aminophenyl)am...
Embodiment 1
[0055] Triamino-tri-triazol (melem) (0.0436 g, 0.2 mmol), 1,3,5-trialdehyde phloroglucinol (0.084 g, 0.4 mmol) and 2,4,6-tri( 4-Aminophenyl)-1,3,5-triazine (0.0709 g, 0.2 mmol) was added to a solvent of 5 mL DMSO. React at 150°C for 12h. First react at 80°C for 6h, then raise the temperature to 120°C for 12h. After the reaction was completed, filter with suction and wash with methanol to remove residual oligomers and the like. The solid was collected and dried under vacuum to obtain 0.173 g of black powder with a yield of 98%.
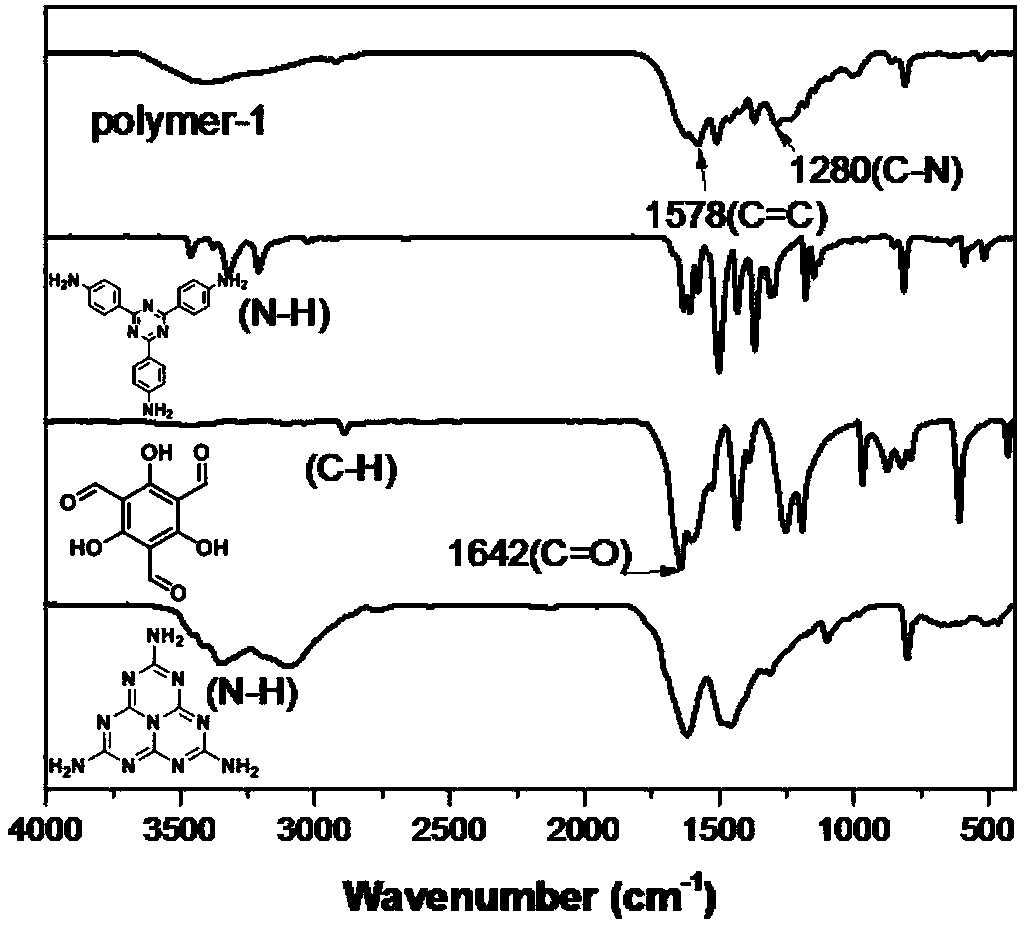
[0056] attached figure 1 It is the Fourier transform infrared spectrogram of the organic porous material prepared in embodiment 1, and the material structural formula is as shown in (1), wherein R 1 to R 6 Each is independently a triazahexacyclyl group represented by formula (6) or a 2,4,6-tris(4-phenyl)-1,3,5-triazinyl group represented by formula (2). Its building block melem is located at 3344cm -1 and 3097cm -1 The amino characteristic peak...
Embodiment 2
[0063] Add 1,3,5-trialdehydephloroglucinol (0.042 g, 0.2 mmol) and triamino-tri-triazol (melem) (0.0436 g, 0.2 mmol), to 5 mL of dimethylformamide in the solvent. React at 80°C for 12h. After the reaction is complete, filter with suction and wash with methanol to remove residual oligomers and the like. The solid was collected and dried under vacuum to obtain 0.060 g of black powder with a yield of 40%.
[0064] attached Figure 10 It is the Fourier transform infrared spectrogram of the organic porous material prepared in Example 2. The material structural formula is shown in (1), wherein R 1 to R 6 All are triazahexacyclyls shown in formula (6). It can be seen from the figure that the building unit melem is located at 3344cm -1 and 3097cm -1 The amino characteristic peak at disappears; 1,3,5-trialdehyde phloroglucinol is located at 2894cm -1 The carbon-hydrogen bond characteristic peak at 1645cm -1 The characteristic peak of the carbonyl at the disappearance indicates...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com