Cardiac troponin I magnetic particle chemiluminescence assay kit and usage method thereof
A cardiac troponin and chemiluminescence detection technology, applied in the field of troponin assay kits, can solve the problems of insufficient sensitivity and anti-interference ability, low degree of automation, low sensitivity, etc., so as to improve detection safety and accuracy, High detection sensitivity and specificity, reducing the effect of manual operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]This embodiment provides a cardiac troponin I magnetic particle chemiluminescence detection kit, which includes a reagent strip consisting of several wells arranged side by side. The reagents contained in the reagent strip include cTnI magnetic particle reagent, cTnI labeled reactant, sample The diluent, calibrator, magnetic bead cleaning solution, and pre-excitation solution, each reagent is sealed and stored in the corresponding hole using heat-sealing film technology, which effectively prevents the reagent from crossing holes and solves the transportation problem.
[0046] On the magnetic particle chemiluminescence detection kit, a magnetic rod cover for use with a special up-suction magnetic bead transfer gun is provided in a hole on the kit, and the kit is used to detect cardiac troponin When detecting magnetic particle chemiluminescence, use the up-suction magnetic bead transfer gun to transfer the magnetic beads, and insert the magnetic rod deep into the liquid to ...
Embodiment 2
[0050] This embodiment provides a preparation method of the magnetic particle chemiluminescence detection kit, which specifically includes the following steps:
[0051] (1) Preparation of Cardiac Troponin I Calibrator
[0052] The cardiac troponin I antigen was prepared into a calibrator solution with 2% BSA and freeze-dried, and the enterprise standard product was used for assignment, and the concentrations were 0.2 and 10 ng / mL;
[0053] (2) Preparation of biotin-labeled cardiac troponin I antibody
[0054] Take 1 mg of cardiac troponin I antibody and dialyze with PB buffer (pH 8.1) at 4°C or room temperature for no less than 16-24 hours; add 0.05 mg of biotin to the dialyzed antibody, and then add PB buffer (pH 8.1) Make the final antibody concentration 1mg / mL, mix for 5min; place the mixture in a constant temperature incubator at 25±1°C, and let it stand for 120±10min to react. Use PB buffer (pH 7.4) to dialyze the biotinylated antibody at 4°C or room temperature for 16-...
Embodiment 3
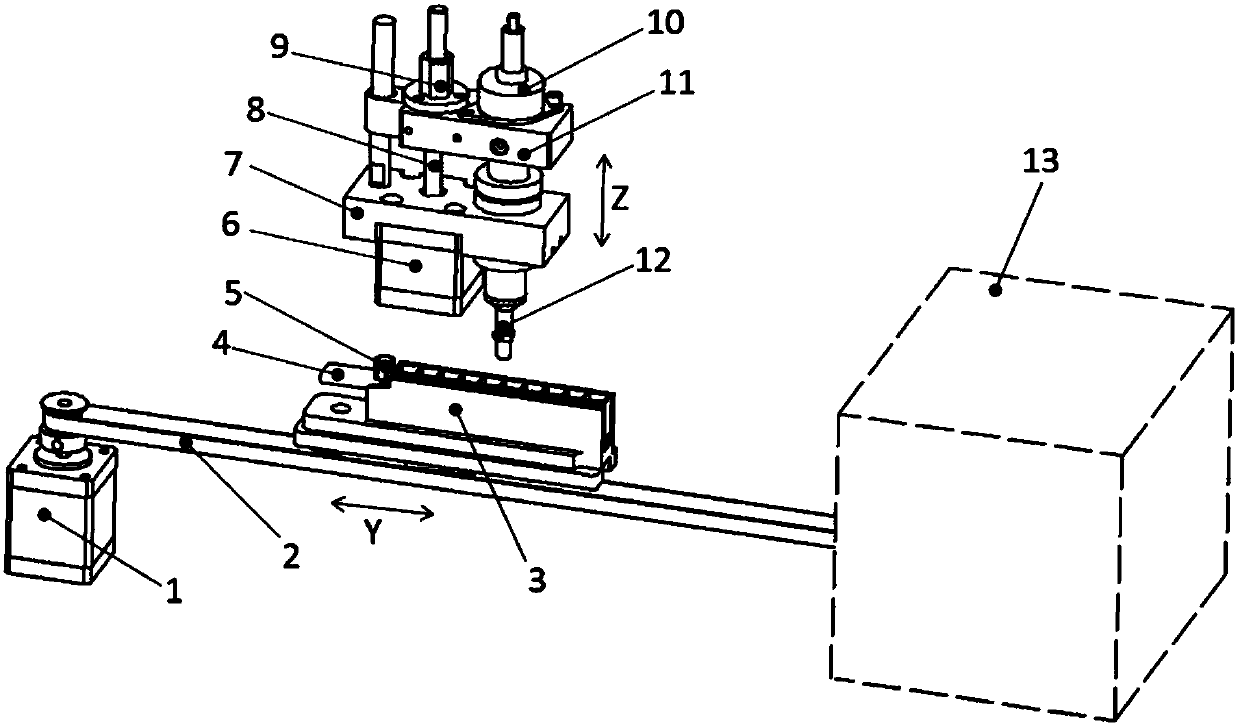
[0074] This embodiment provides a method for using the cardiac troponin I magnetic particle chemiluminescence detection kit. During specific detection, the reagent strip 4 is placed in the incubation chamber 3, and the incubation chamber 3 is arranged on the conveyor belt. 2, the conveyor belt 2 is connected to the horizontal displacement motor 1, and the end of the conveyor belt 2 is provided with an analysis reading module 13; and an up-suction magnetic bead transfer gun that can move up and down relative to the magnetic rod cover 5 is used to realize the magnetization on the reagent strip. Rod cover, suction magnetic beads, magnetic bead elution, magnetic bead mixing, magnetic rod cover removal action;
[0075] Specifically include the following steps:
[0076] Step 1, take out the reagents, and place them in room temperature environment away from light as required. The reagent strips should be kept in an opaque aluminum foil bag. If you need to take them out, it is recomme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com