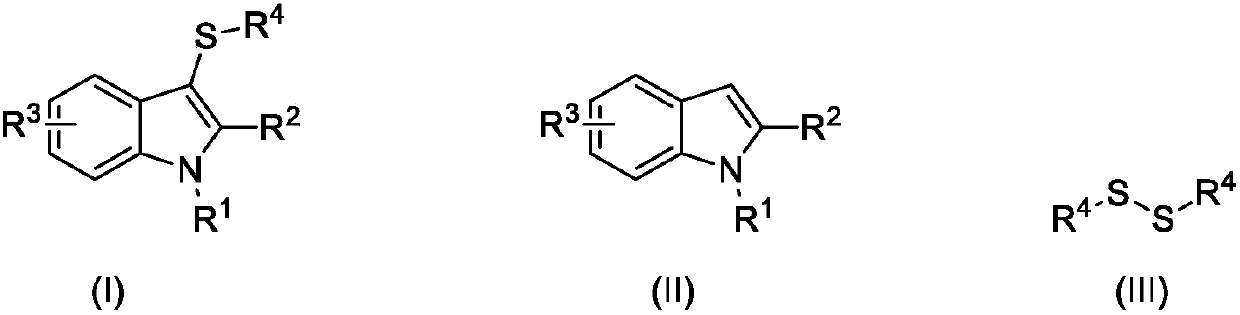
Synthesizing method for 3-mercapto indole compound through electrochemical catalytic oxidation
A technology of mercaptoindole and catalytic oxidation, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of non-progress of reaction and mercaptan peroxidation, and achieve the effect of reducing environmental costs and easy and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
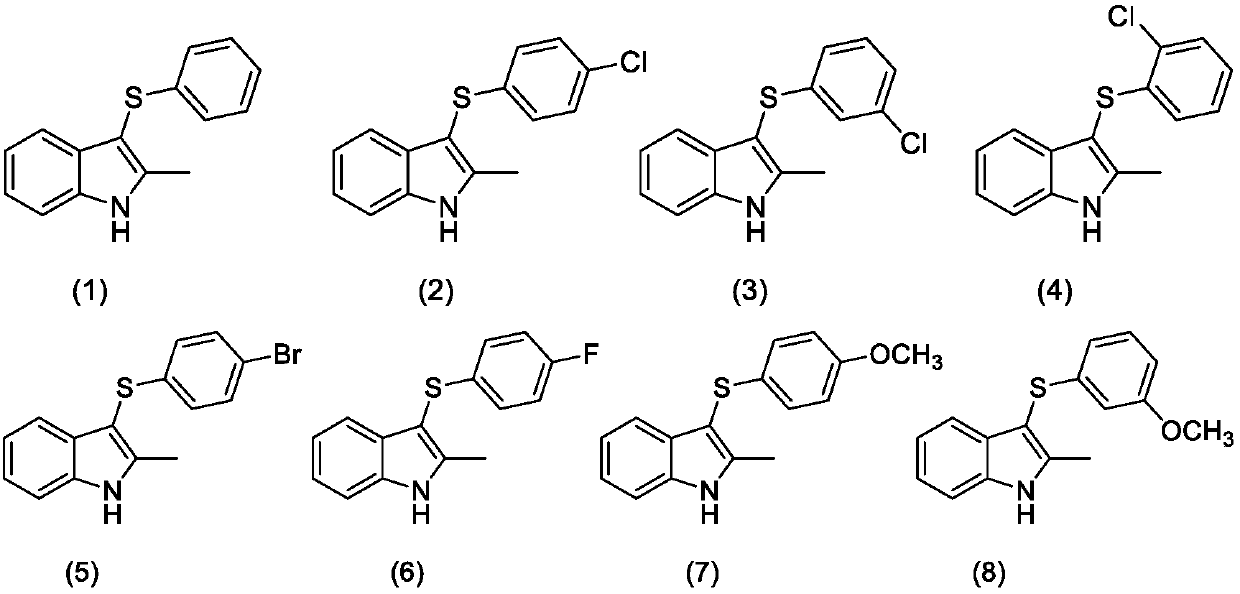
[0025] Embodiment 1: the preparation of 2-methyl-3-phenylthio-1H-indole (formula 1)
[0026] Add 0.1mol / L sodium tetrafluoroborate in acetonitrile solution (15mL), 2-methyl-1H-indole (1mmol), diphenyl disulfide (0.5mmol) and potassium iodide (0.05mmol) into a 30ml beaker . 60°C, constant potential electrolysis at 0.4V, the reaction ends after 6h. The solvent was evaporated under reduced pressure, and then separated by column chromatography, using a mixture of ethyl acetate / n-hexane with a volume ratio of 1:100 as the eluent, the eluate containing the target compound was collected, and the solvent was evaporated to obtain the product 2- Methyl-3-phenylthio-1H-indole. The isolated yield was 94%.
Embodiment 2
[0027] Embodiment 2: the preparation of 2-methyl-3-phenylthio-1H-indole (formula 1)
[0028] The reaction steps were the same as in Example 1, except that the voltage was changed to 0.6V, and the reaction was performed for 5 hours. The isolated yield of 2-methyl-3-phenylthio-1H-indole was 94%.
Embodiment 3
[0029] Embodiment 3: the preparation of 2-methyl-3-phenylthio-1H-indole (formula 1)
[0030] The reaction steps are the same as in Example 1, except that the voltage is changed to 0.2V, and the reaction takes 24 hours. The isolated yield of 2-methyl-3-phenylthio-1H-indole is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com