Method for preparing methyl glycolate and haloalkane as byproduct
A technology of methyl glycolate and halogenated alkanes is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of affecting catalytic reaction performance, low conversion rate of raw materials, low product selectivity, etc., Achieve the effect of raw material conversion rate and high product selectivity, no environmental pollution, and short reaction path.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
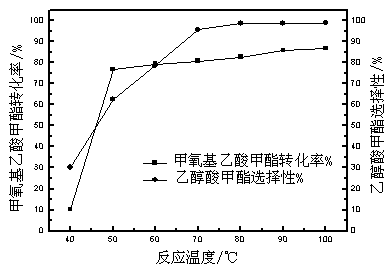
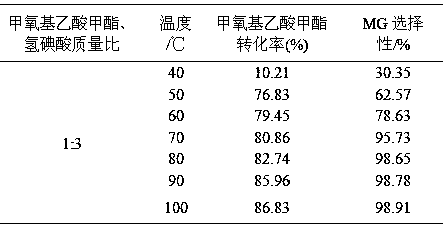
[0047] Weigh 200g of methyl methoxyacetate and 600g of hydroiodic acid (mass ratio 1:3) into the reaction kettle, and react at 40, 50, 60, 70, 80, 90, and 100°C for 5 hours respectively. The results are shown in Table 1.
[0048] Table 1 Effects of different reaction temperatures on the conversion of raw materials and product selectivity
[0049]
[0050] It can be seen from Table 1 that as the reaction temperature rises, the conversion rate of methyl methoxyacetate increases gradually, and the selectivity of MG also gradually increases. After 70°C, as the temperature rises, the selectivity of MG is basically stable and maintains at 98 ~99% range.
Embodiment 2
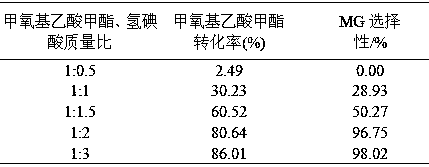
[0052] Under the condition of 80°C, methyl methoxyacetate and hydroiodic acid were added into the reactor according to the mass ratio of 1:0.5, 1:1, 1:1.5, 1:2, 1:3, and reacted for 5 hours. The reaction results are shown in the table 2.
[0053] Table 2 Effects of different raw material mass ratios on raw material conversion and product selectivity
[0054]
[0055] As can be seen from Table 2, as the mass ratio of methyl methoxyacetate to hydroiodic acid decreases, the conversion rate of methyl methoxyacetate increases and the selectivity of MG increases. The optimum mass ratio of iodic acid is 1:3.
Embodiment 3
[0057] Weigh 200 g of methyl methoxyacetate and 600 g of hydroiodic acid (mass ratio 1:3) into the reactor, and react at 80 °C for 0.5, 1, 2, 5, and 10 hours. The reaction results are shown in the table 3.
[0058] Table 3 Effects of different reaction times on the conversion of raw materials and product selectivity
[0059]
[0060] It can be seen from Table 3 that as the reaction time increases, the conversion rate of methyl methoxyacetate gradually increases, and the selectivity of MG first increases and then decreases. After 5 h, the selectivity of MG is the highest at 98.73%. The conversion rate of ethylene glycol monomethyl ether is basically stable, and the mass selectivity of MG tends to be stable at about 98%. So the best time for the reaction is 5h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com