A kind of wheat spike-shaped porous magnesium oxide and its preparation method
A porous magnesia and wheat ear-shaped technology, applied in the direction of magnesia, etc., can solve the problems of difficult separation and treatment of organic solvents, increase the difficulty of waste liquid treatment, and few research reports, and achieve rich surface pores, fast adsorption rate, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
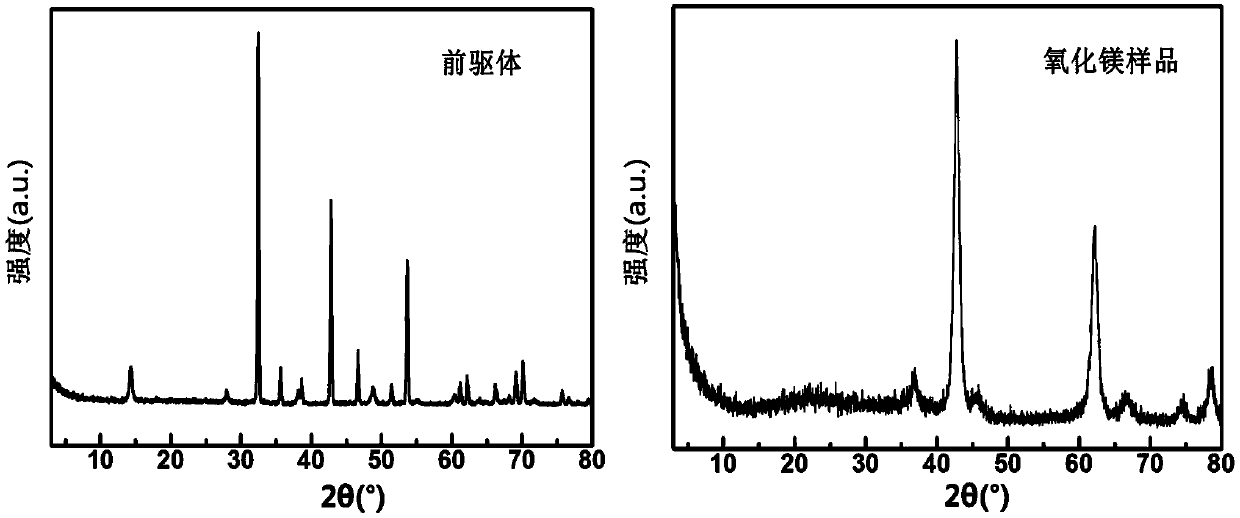
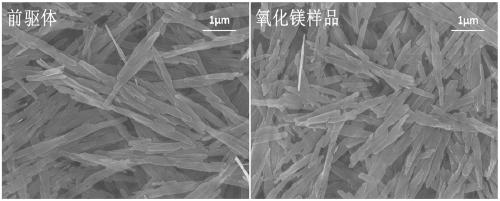
Image
Examples
Embodiment 1
[0029] Step 1: Ultrasonic dissolve 4.1g magnesium chloride hexahydrate and 1.2g aluminum chloride hexahydrate in 50mL deionized water, where Mg 2+ The concentration is 0.2mol / L; Al 3+ The concentration is 0.05mol / L, Al 3+ With Mg 2+ The ratio is 0.25; then 6.0g of urea is ultrasonically dissolved in 50mL of deionized water, and the two completely dissolved solutions are mixed and stirred evenly;
[0030] Step 2: Transfer the solution obtained in step 1 to a hydrothermal reactor, react at 140°C for 12 hours, with a stirring speed of 500r / min, naturally cool to room temperature, centrifuge and wash with deionized water to neutral;
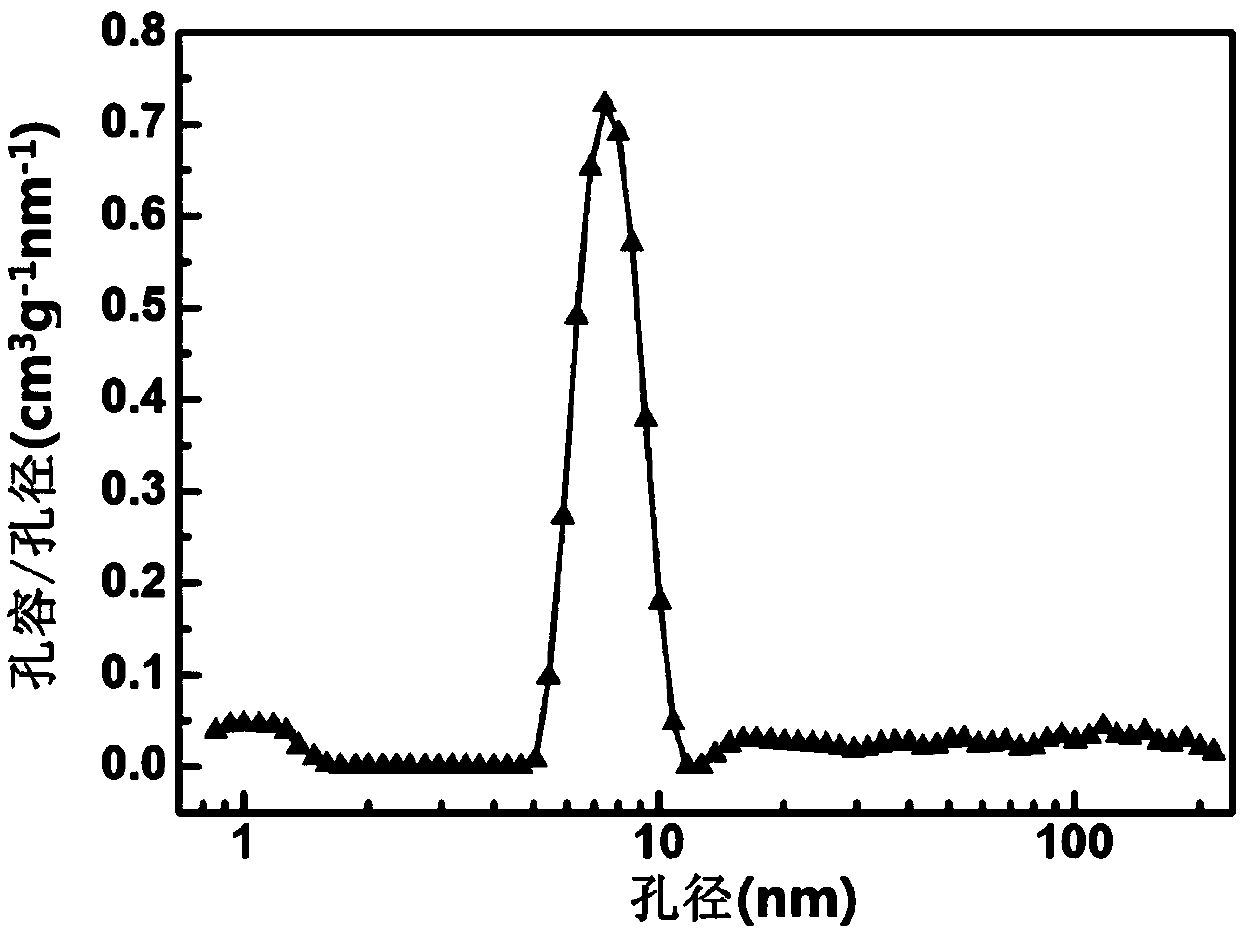
[0031] Step 3: Dry the filter cake obtained above at 80°C for 12h, grind the obtained sample to powder, set the heating rate to 5°C / min, calcination temperature 550°C, and calcination time 3h in an air atmosphere in a muffle furnace , It is calcined to obtain magnesium oxide. Its specific surface area is 95.28m 2 / g, the pore volume is 0.19cm 3 / g.
[0032...
Embodiment 2
[0039] Step 1: Dissolve 5.1g magnesium nitrate hexahydrate and 3.8g aluminum nitrate nonahydrate ultrasonically in 50mL deionized water (Mg 2+ The concentration is 0.2mol / L, Al 3+ The concentration is 0.1mol / L, Al 3+ With Mg 2+ The ratio is 0.5), and then ultrasonically dissolve 7.0g of hexamethylenetetramine in 50mL of deionized water, and then mix the two completely dissolved solutions and stir evenly;
[0040] Step 2: Transfer the solution obtained in step 1 to a hydrothermal reactor, react at 160°C for 12 hours, with a stirring speed of 500r / min, naturally cool to room temperature, centrifuge, and wash with deionized water until neutral;
[0041] Step 3: Dry the filter cake obtained above at 80°C for 12 hours, grind the obtained sample to powder, set the heating rate to 10°C / min, calcination temperature 500°C, and calcination time 4h in an air atmosphere in a muffle furnace , It is calcined to obtain magnesium oxide. Its specific surface area is 132.5m 2 / g, the pore volume is ...
Embodiment 3
[0044] Step 1: Dissolve 4.9g magnesium sulfate heptahydrate and 6.7g aluminum sulfate octahydrate ultrasonically in 50mL deionized water (Mg 2+ The concentration is 0.2mol / L, Al 3+ The concentration is 0.02mol / L, Al 3+ With Mg 2+ The ratio is 0.1), and then ultrasonically dissolve 11.5 g of ammonium carbonate in 50 mL of deionized water, and then mix the two completely dissolved solutions and stir them evenly;
[0045] Step 2: Transfer the solution obtained in step 1 to a hydrothermal reactor, react at 180°C for 12 hours, with a stirring speed of 600r / min, naturally cool to room temperature, centrifuge and wash with deionized water to neutral;
[0046] Step 3: Dry the filter cake obtained above at 80°C for 12h, grind the obtained sample to powder, set the heating rate 5°C / min, calcination temperature 600°C, and calcination time 3h in an air atmosphere in a muffle furnace , It is calcined to obtain magnesium oxide. Its specific surface area is 87.3m 2 / g, the pore volume is 0.18cm 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Equilibrium adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com