Medicine capable of controlling feline distemper as well as preparation method and applications of medicine
A technology of feline distemper and medicine, which is applied in the field of pet medicine, can solve the problems such as feline distemper cannot be cured, and achieve the effect of convenient long-distance transportation, broad application prospects, and potential harm of price reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, culture of human umbilical cord mesenchymal stem cells and collection of culture supernatant
[0030] 1. Preparation of human umbilical cord mesenchymal stem cells
[0031] The preparation of human umbilical cord mesenchymal stem cells was performed in an ultra-clean bench, and the following steps were used:
[0032] (1) Collect the umbilical cord tissue of a healthy person, and use normal saline to wash away the residual blood in the tissue and blood vessels;
[0033] (2) Separating and removing the umbilical cord vein, artery and amniotic tissue;
[0034] (3) Shred the gelatinous part of the umbilical cord tissue in step (2) to 1-2 mm 3 Tissue block, using IMDM medium (purchased from gibco) digestion solution containing 0.1% (M / V) collagenase I (purchased from sigma) and 0.25% trypsin (purchased from armesco) at 37 degrees, 100 rpm shaker digestion 2 hours;
[0035] (4) Centrifuge at 2500rpm for 10 minutes, remove the supernatant, and wash the precip...
Embodiment 2
[0040] Example 2, Collection of Bioactive Factors in Human Umbilical Cord Mesenchymal Stem Cell Culture Supernatant The steps of collecting bioactive factors in human umbilical cord mesenchymal stem cell culture supernatant include:
[0041] (1) Place the supernatant stored at -20°C collected in Example 1 at 4°C overnight to melt;
[0042] (2) Centrifuge at 2500rpm for 10 minutes, remove cell debris, and collect the culture supernatant of human umbilical cord mesenchymal stem cells;
[0043] (3) Use a stirring ultrafiltration cup (purchased from millipore) to carry out ultrafiltration. The ultrafiltration membrane molecular weight cut-off of the stirring ultrafiltration cup is 100Kda, and the human genome fragments, microorganisms and molecular weights exceeding 100Kda in the culture medium are removed. proteins and other substances; and
[0044] (4) The human umbilical cord mesenchymal stem cell culture supernatant after ultrafiltration is concentrated by ultrafiltration usi...
Embodiment 3
[0046] Embodiment 3, a kind of feline distemper medicine and preparation method thereof
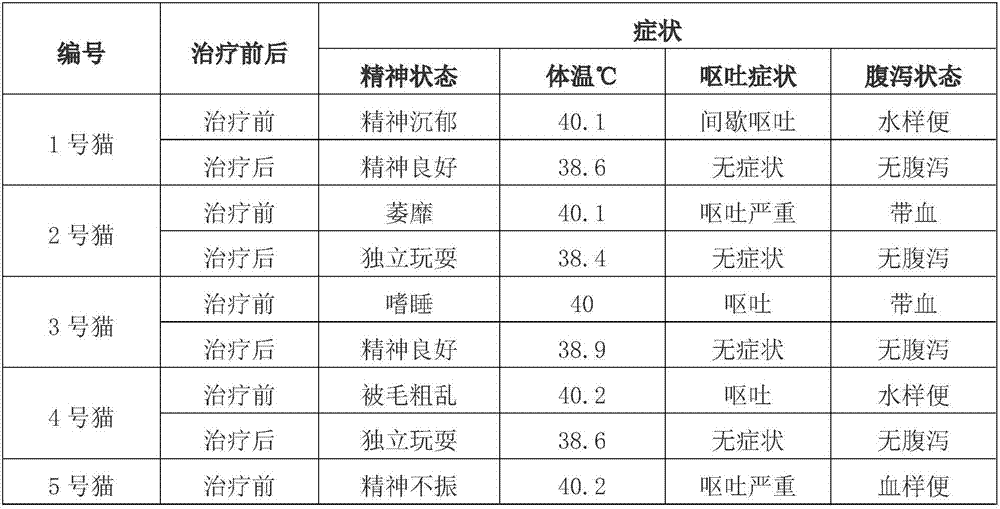
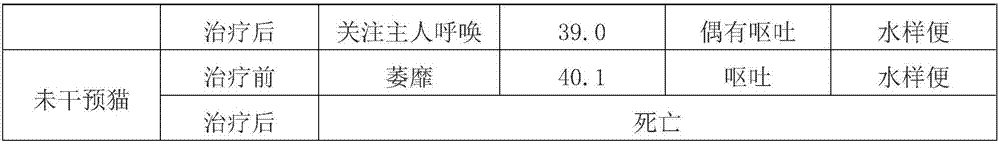
[0047]A medicine for effectively controlling feline distemper, comprising the human umbilical cord mesenchymal stem cell culture supernatant containing bioactive factors collected in Example 2, recombinant feline ω-interferon, granulocyte colony-stimulating factor and water for injection. The molecular weight of the bioactive factor is 3-100Kda, the protein concentration of the bioactive factor in the human umbilical cord mesenchymal stem cell culture supernatant is 1-5 mg / mL, and the concentration of the recombinant feline ω-interferon is 5 mg / mL. ~200,000 U / mL, the concentration of the granulocyte colony-stimulating factor is 20-30 μg / mL. The optimum protein concentration of bioactive factors in the human umbilical cord mesenchymal stem cell culture supernatant is 2 mg / mL, the optimum concentration of the recombinant cat ω-interferon is 100,000 U / mL, and the granulocyte colony The conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com