Nitrogen-containing heterocycle derivatives and applications thereof
A technology for heterocyclic groups and compounds, applied to nitrogen-containing heterocyclic derivatives and their application fields, can solve the problems of only using injection preparations, high cost, etc., and achieve the effects of enhancing uptake ability, low manufacturing cost, and increasing expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0136] The general method of the present invention is further explained below. The compound of the present invention can be prepared by methods known in the art. The preparation process of the preferred compound of the present invention is used as an example to illustrate in detail below, but the preparation method of the compound of the present invention is not limited thereto. .
[0137] The preparation of the general formula compound (V) disclosed by the present invention is mainly carried out with reference to the following schemes,
[0138]
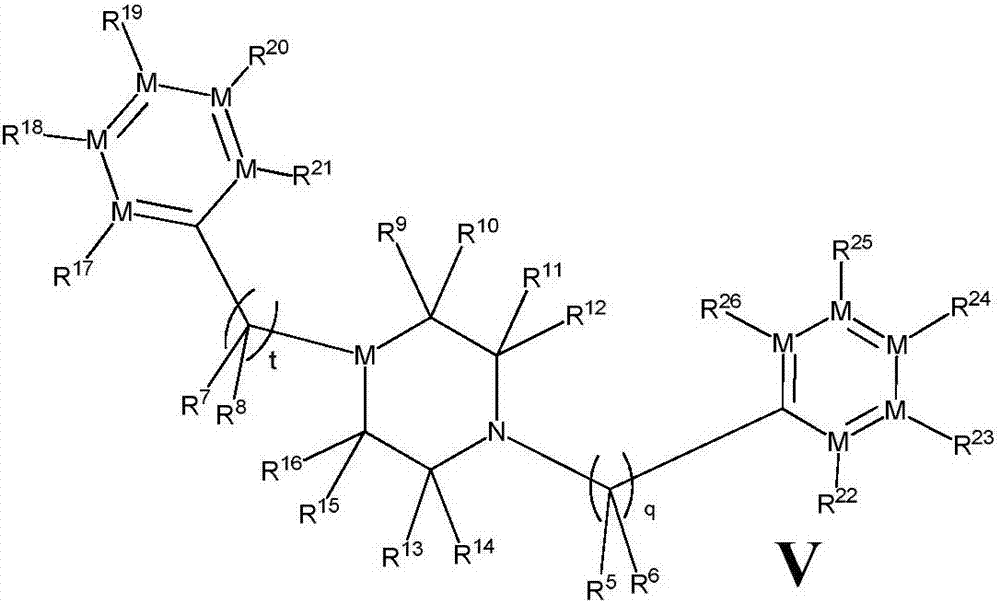
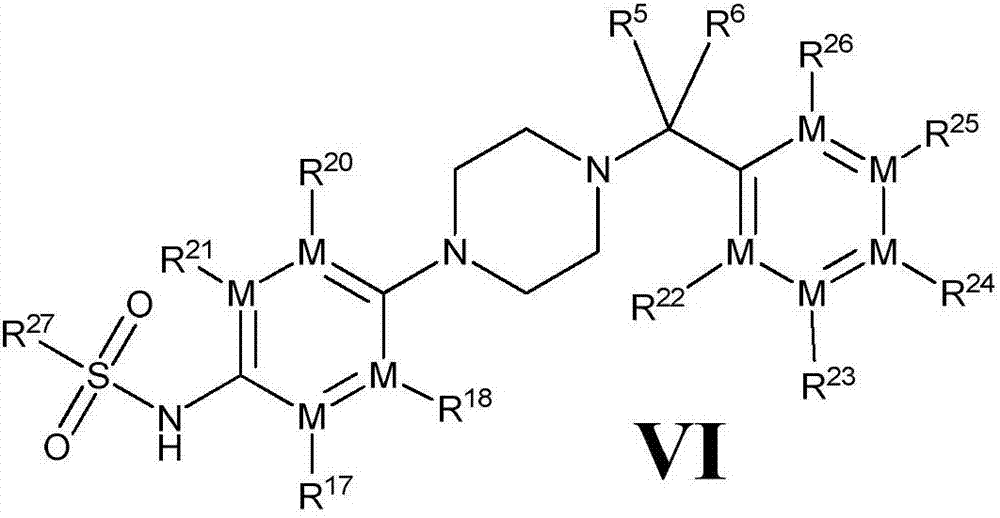
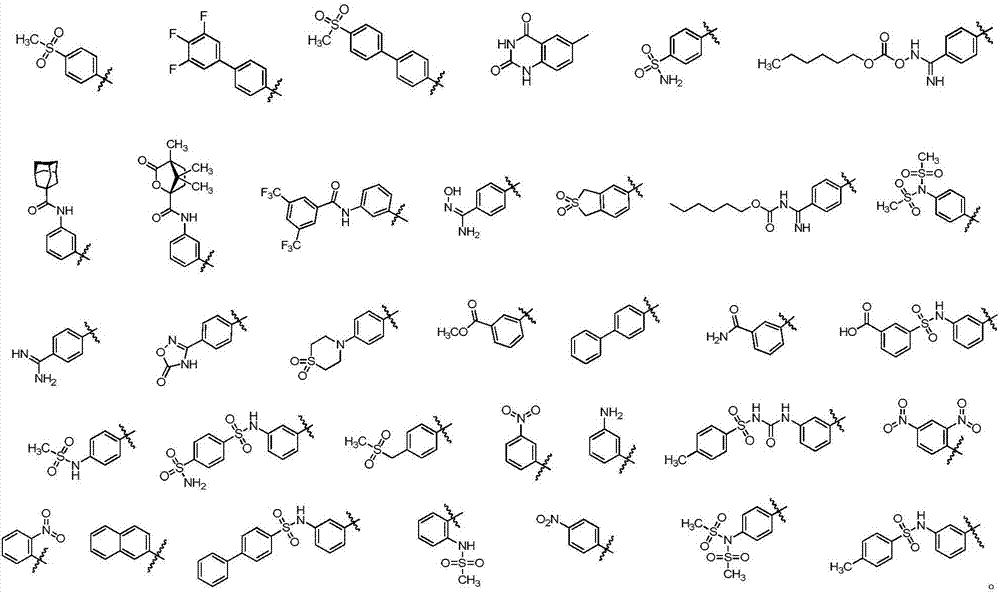
[0139] The compound represented by the general formula (V) of the present invention, its stereoisomers, its tautomers, its solvates and pharmaceutically acceptable salts thereof, wherein a preparation method comprises the following steps :
[0140]
[0141] X5 and X8 are the starting materials of this scheme, which can be obtained from commercially available products, or prepared according to methods reported in literature. C...
Embodiment 1
[0159]
[0160] Compound 1A (150 mg, 0.458 mmol) was dissolved in dichloromethane (20 mL), then triethylamine (70 μL, 0.504 mmol) was added, stirred at room temperature, and 1B (128 mg, 0.504 mmol) was added in batches, and the addition was completed. React overnight at room temperature. The reaction solution was spin-dried, added saturated aqueous sodium bicarbonate solution and ethyl acetate, extracted and separated, collected the organic phase, dried over anhydrous sodium sulfate, spin-dried, added dichloromethane to the obtained residue, precipitated a solid, stirred for 30 min, and suction filtered. After washing with dichloromethane, the filter cake was collected and dried to obtain compound 1 as a white solid (61 mg, yield 24.4%). 1 H NMR (400MHz, DMSO-d 6 )δ10.10(s,1H),8.14–8.07(m,2H),7.96–7.89(m,2H),6.95–6.86(m,4H),6.85–6.77(m,3H),3.73(s, 6H),3.42(s,2H),3.29(s,3H),3.10–2.99(m,4H),2.49–2.40(m,4H).MS(ESI)m / z[M+H] + ,546.1.
Embodiment 2
[0162]
[0163] Compound 1A (600mg, 1.83mmol) was dissolved in dichloromethane (15mL), then triethylamine (286μL, 2.01mmol) was added, stirred at room temperature, and then a solution of 2B (609mg, 2.01mmol) in dichloromethane was added dropwise , After dropping, react overnight at room temperature. The reaction solution was spin-dried, added saturated aqueous sodium bicarbonate solution and ethyl acetate, extracted and separated, collected the organic phase, dried over anhydrous sodium sulfate, spin-dried, added dichloromethane to the obtained residue, precipitated solid, suction filtered, dichloromethane After washing, the filter cake was collected and dried to obtain off-white solid 2C (510 mg, yield 47.0%).
[0164] Add compound 2C (150mg, 0.253mmol) in 25mL round bottom flask, 2D (44mg, 0.253mmol), Pd(dppf) 2 Cl 2 (18mg, 0.025mmol), potassium acetate (74mg, 0.759mmol), THF / EtOH / water (3mL / 3mL / 3mL) mixed solvent, react overnight in an 80°C oil bath under nitrogen prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com