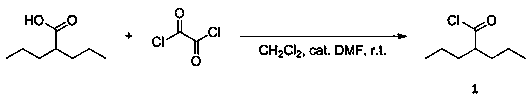A kind of fat-soluble emodin derivative and its preparation method and application
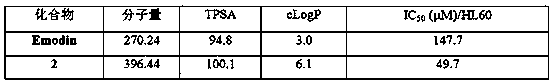
A technology of derivatives and emodin, applied in the field of emodin derivatives and their preparation, can solve problems such as unsatisfactory activity, and achieve the effects of mild experimental conditions and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
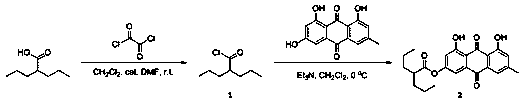
[0024] Preparation of fat-soluble emodin derivatives
[0025] (1) Preparation of 2-propylpentanoyl chloride (compound 1)
[0026]
[0027] 1. Reaction: Take a dry and clean 100 mL eggplant-shaped flask, dissolve 10 mmol 2-propylvaleric acid in 20 mL methylene chloride, and then add 12 mmol oxalyl chloride into the reaction system with a spatula. The mixed solution was stirred under ice bath conditions for 12 hours, and the reaction was stopped after TLC detected that there was no raw material in the system (developing system: dichloromethane:methanol=20:1, volume ratio);
[0028] 2. Purification: Spin dry directly with a rotary evaporator to obtain the crude product, and then purify to obtain 2-propylpentanoyl chloride (compound 1).
[0029] 1 3 δ 2.84 – 2.74 (m, 1H), 1.82 – 1.66 (m, 2H), 1.57 – 1.48 (m, 2H),1.43 – 1.32 (m, 4H), 0.93 (t, J = 7.2 Hz, 6H). 13 C NMR (101 MHz, CDCl 3 ) δ177.55, 56.93, 34.26, 20.35, 13.99. (In the actual production process, this step rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com