Heating solid-phase synthetic method of polypeptides
A solid-phase synthesis and condensation technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., to achieve the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
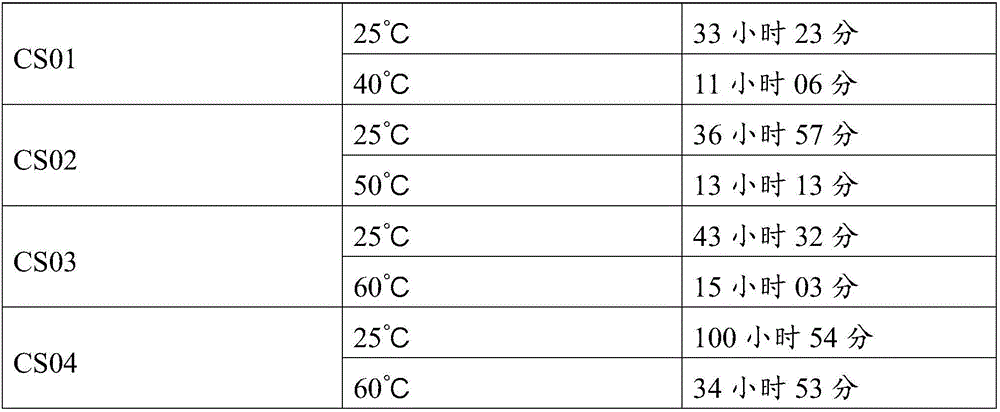
[0041] Embodiment 1: the preparation method of Ala-Ala-Lys-Lys-Ala-Ala-Lys-Lys-Ala-Lys (CS01)
[0042] (1) Heating and condensing amino acids: add polypeptide resin, Fmoc-protected amino acids, condensing agents and solvents to the reactor, heat to the reaction temperature, and the reaction time is 10-30min. After the reaction, use N,N- Wash the resin 3 times with dimethylformamide, each elution time is 20-40s;
[0043] In this embodiment, the reactor is a 3-channel reactor;
[0044] Polypeptide resin is Wang resin in the present embodiment;
[0045] Condensing agent is DIC / HOBt in the present embodiment;
[0046] The solvent in this example is N,N-dimethylformamide.
[0047] (2) Removal of Fmoc by heating: add 20% piperidine / N,N-dimethylformamide solution of deprotecting agent, heat to reaction temperature, reaction time 2-5min, after the reaction is completed, use N at the reaction temperature , washing the resin once with N-dimethylformamide, and then washing the resin ...
Embodiment 2
[0056] Embodiment 2: the preparation method of Ile-Asp-Leu-Leu-Gln-Gly-Arg-Thr-Arg-Asn-Arg-Cys (CS02)
[0057](1) Heating and condensing amino acids: add polypeptide resin, Fmoc-protected amino acids, condensing agents and solvents to the reactor, heat to the reaction temperature, and the reaction time is 10-30min. After the reaction, use N,N- Wash the resin 3 times with dimethylformamide, each elution time is 20-40s;
[0058] In this embodiment, the reactor is a 3-channel reactor;
[0059] Polypeptide resin is chlorine resin in the present embodiment;
[0060] Condensing agent is DIC / HOAt in the present embodiment;
[0061] The solvent in this example is N,N-dimethylformamide.
[0062] (2) Removal of Fmoc by heating: add 20% piperidine / N,N-dimethylformamide solution of deprotecting agent, heat to reaction temperature, reaction time 2-5min, after the reaction is completed, use N at the reaction temperature , washing the resin once with N-dimethylformamide, and then washing...
Embodiment 3
[0072] The preparation method of Ala-Tyr-Arg-Ala-Ile-Arg-His-Ile-Pro-Arg-Arg-Ile-Arg-Gln (CS03)
[0073] (1) Heating and condensing amino acids: add polypeptide resin, Fmoc-protected amino acids, condensing agents and solvents to the reactor, heat to the reaction temperature, and the reaction time is 10-30min. After the reaction, use N,N- Wash the resin 3 times with dimethylformamide, each elution time is 20-40s;
[0074] In this embodiment, the reactor is a 3-channel reactor;
[0075] In this embodiment, the polypeptide resin is an amino acid resin;
[0076] Condensing agent is DIEA / HBTU in the present embodiment;
[0077] The solvent in this example is chloroform.
[0078] (2) Removal of Fmoc by heating: add 20% piperidine / N,N-dimethylformamide solution of deprotecting agent, heat to reaction temperature, reaction time 2-5min, after the reaction is completed, use N at the reaction temperature , washing the resin once with N-dimethylformamide, and then washing the resin o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com