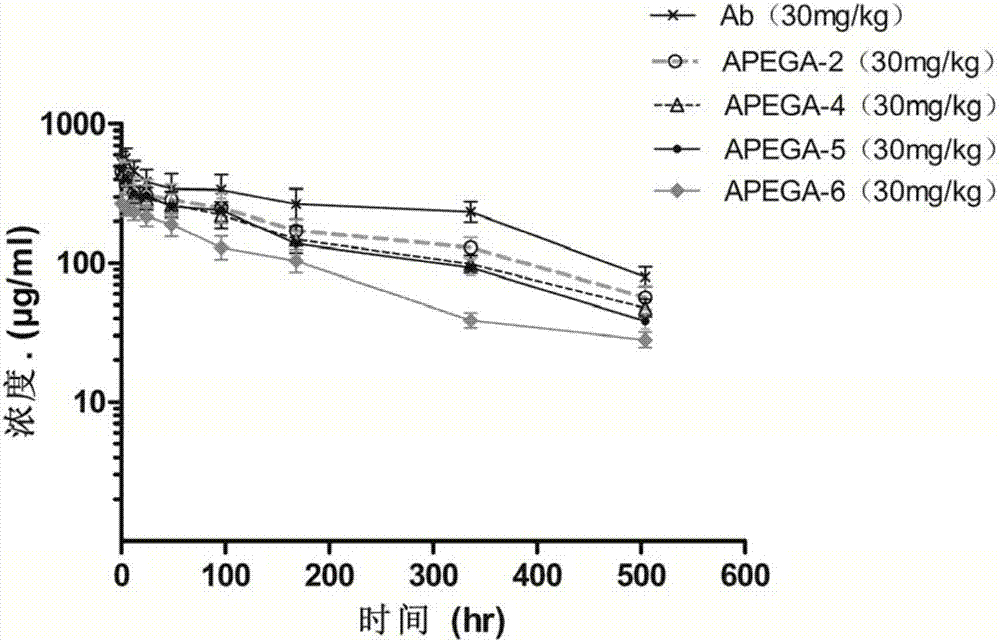
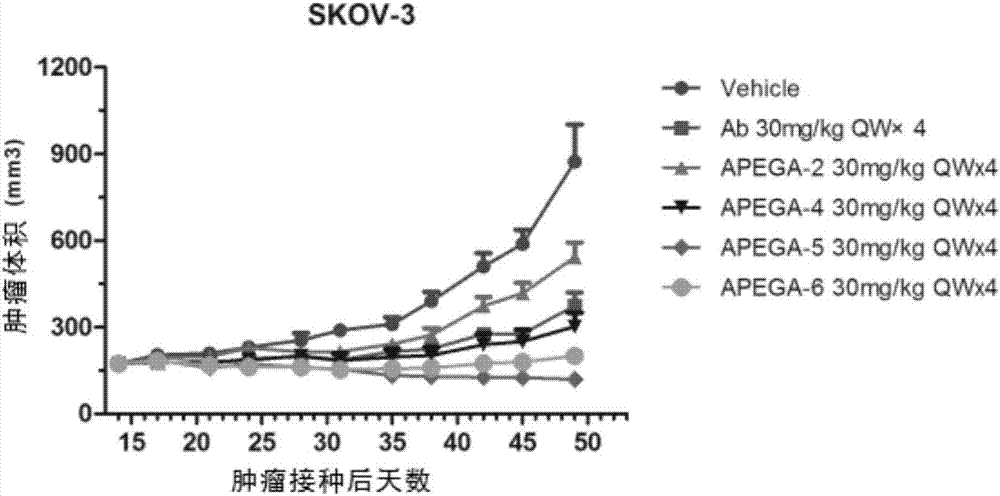
PEG connecter and ligand drug conjugates
A technology of drug conjugates and linkers, used in drug combinations, anti-infective drugs, pharmaceutical formulations, etc., can solve problems such as limiting activity, accelerating the efflux of hydrophobic compounds, and increasing activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Embodiment 1: Synthesis of four-arm polyethylene glycol hydroxy-monoacetic acid (III-1)
[0157]
[0158] step:
[0159] In a three-necked round-bottomed flask, pass nitrogen gas, add 100g of four-armed polyethylene glycol (4ARM-PEG-5K) and 800mL of tetrahydrofuran (THF), heat to dissolve, evaporate about 20% of the solvent, cool down, and add 4.48g of potassium tert-butoxide , reacted at room temperature for 2 hours, added dropwise 5.17mL tert-butyl bromoacetate, reacted overnight at room temperature, filtered the next day, concentrated the reaction solution to viscous, added 500mL alkaline solution (add 8.16g sodium hydroxide and 77.52g sodium hydroxide to 500mL water Sodium phosphate), alkali hydrolysis at 80°C for 2 hours, adjust the pH of the solution to 2-3 with 2N hydrochloric acid solution, add 15% sodium chloride to the solution, extract three times with dichloromethane, combine the organic phases, and dry with anhydrous sodium sulfate , filtered, concentra...
Embodiment 2
[0162] Embodiment 2: Four-arm polyethylene glycol hydroxy-methyl monoacetate (IVA-1)
[0163]
[0164] step:
[0165] In a single-necked round-bottomed flask, add 3.2g of four-armed polyethylene glycol hydroxy-monoacetic acid (III-1), dissolve it with 16mL of anhydrous methanol, put it in an ice-water bath, add 0.64mL of concentrated sulfuric acid dropwise, and react at room temperature for 3 hours. % sodium bicarbonate aqueous solution to adjust the pH of the system to 7.0, extract three times with dichloromethane, combine the organic phases, dry the organic phases with anhydrous magnesium sulfate, filter, concentrate at 40°C until viscous, precipitate with ether, and dry in vacuo to obtain four arms Polyethylene glycol hydroxy-methyl monoacetate (IVA-1).
[0166] NMR(DMSO)δ: 3.32(s,3H,CH 2 COOCH 3 ), 4.13 (s,2H,CH 2 COOCH 3 ), 4.57(t,3H,CH 2 OH).
Embodiment 3
[0167] Embodiment 3: four-arm polyethylene glycol sulfonate-methyl monoacetate (IVB-1)
[0168]
[0169] step:
[0170] In a three-neck round bottom flask, nitrogen gas was added, 3.0 g of four-armed polyethylene glycol hydroxy-methyl monoacetate was added, dissolved in 50 mL of toluene, 38 mL of toluene was evaporated by heating until the distillate was clear, and cooled to room temperature, and 5 mL of dichloro methane, stirred for 10 minutes, added 188 μL of triethylamine, stirred for 5 minutes, added dropwise 94 μL of methanesulfonyl chloride, sealed and reacted overnight, the next day, added 720 μL of absolute ethanol, stirred for 15 minutes, filtered, concentrated at 60°C until viscous, Dissolve in 60 mL of isopropanol and precipitate in an ice-water bath, filter, wash the filter cake once with isopropanol, and dry in vacuo to obtain four-armed polyethylene glycol sulfonate-methyl monoacetate (IVB-1).
[0171] NMR(DMSO)δ: 3.17(s,9H,CH 2 OSO 2 CH 3 ), 4.13 (s,2H,CH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com