Intravenous ganaxolone formulations and their use in treating status epilepticus and other seizure disorders
A technology of ganaxolone and preparations, applied in the field of intravenous ganaxolone preparations and its use in the treatment of status epilepticus and other seizure disorders, which can solve the problems of unapproved treatment of epilepsy in children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1. Preparation of Injectable Ganaxolone Formulations
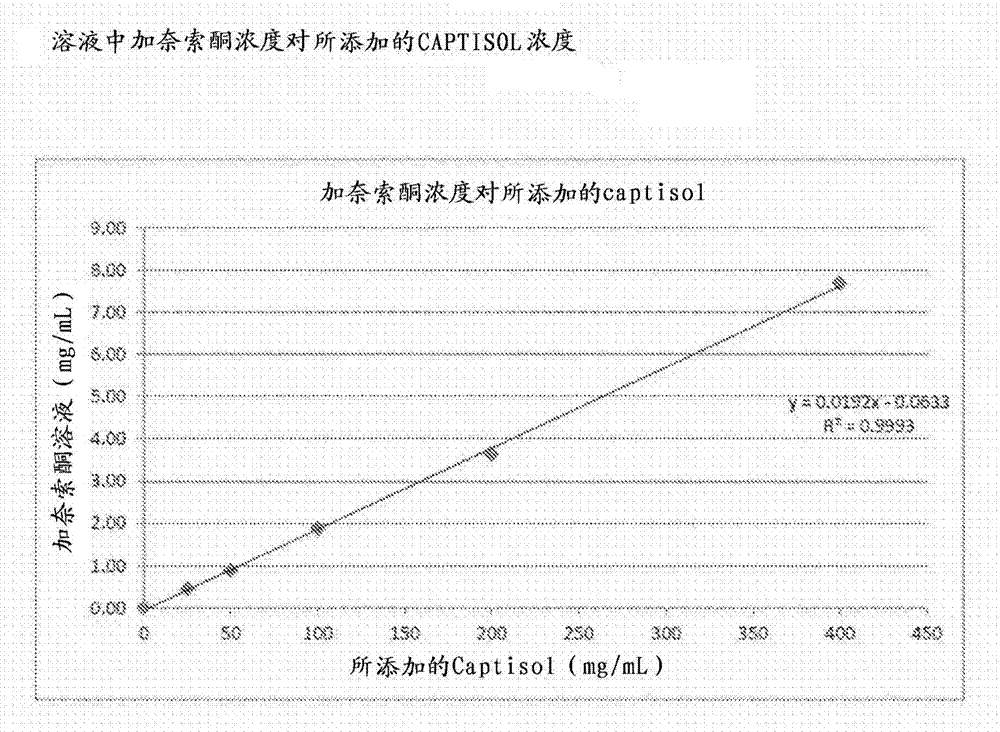
[0114] Firstly, the solubility of ganaxolone in CAPTISOL aqueous solution was determined by constructing a phase solubility diagram. Equilibrium was achieved by shaking excess ganaxolone in an aqueous solution of known concentration of CAPRISOL for 42 hours. The ganaxolone solution was filtered through a 0.45 μm syringe filter into an HPLC vial. The ganaxolone concentration of the filtrate was determined by HPLC. The results are summarized in Table 1. The moles of ganaxolone in solution are plotted against the moles of CAPTISOL added. The solubility of ganaxolone in water was found to increase linearly with the addition of CAPTISIOL, indicating the formation of a 1:1 complex between ganaxolone and CAPTISOL. The graph of the weight (mg) of ganaxolone in the solution relative to the weight (mg) of ganaxolone added ( figure 1 ) shows that the weight:weight ratio of CAPTISOL to ganaxolone required for ganax...
Embodiment 2
[0117] Example 2. Preparation of Injectable Ganaxolone-CAPTISOL Solution (5mg / mL)
[0118] Ganaxolone (0.50 g) was first mixed manually with a small amount (approximately 20 mL) of 30% w / v CAPTISOL solution in sterile water for injection using a spatula to form a homogeneous paste. An additional amount (about 40 mL) of 30% w / v CAPTISOL solution was then added to obtain a slurry. The suspension was stirred for 20 minutes with a magnetic stir bar. Sonicate it for 2 hours using a probe sonicator. While sonicating, an additional 30% w / v CAPTISOL solution was added until the total amount of CAPTISOL solution reached 99.58 mL. The stirred formulation was then heated at 68.5°C for about 2.5 hours to obtain a solution. The heat was removed and the solution was stirred at room temperature for about 2 hours. Replace volume lost due to evaporation with water. The clear solution was sterile filtered through a 0.2 μm nylon membrane.
Embodiment 3
[0119] Example 3. Preparation of lyophilized Ganaxolone-CAPTISOL powder
[0120] Prepare Ganaxolone by dissolving 42.6 mg of Ganaxolone in 6.6 mL of 40% w / v CAPTISOL and stirring for 1 hour (a small amount of undissolved Ganaxolone is filtered through a 0.45 μm syringe filter to obtain a clear solution) Xolone-CAPTISOL solution. The solution was frozen in a dry ice / acetone bath and lyophilized for 2 days to obtain 2.859 g of a free flowing white powder. The ganaxolone concentration of the lyophilized powder was determined by HPLC to be 1.26% by weight, which is slightly lower than the theoretical value (1.49% by weight). The lyophilized powder was reconstituted in water to obtain a clear solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com