Phage-assisted multi-bacterial continuous directed evolution system and method
A directed evolution and phage technology, applied in biochemical equipment and methods, viruses/phages, bacteria, etc., can solve the problems of mutual interference of different genetic elements and low efficiency of phage proliferation and evolution, and achieve the effect of improving evolution efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
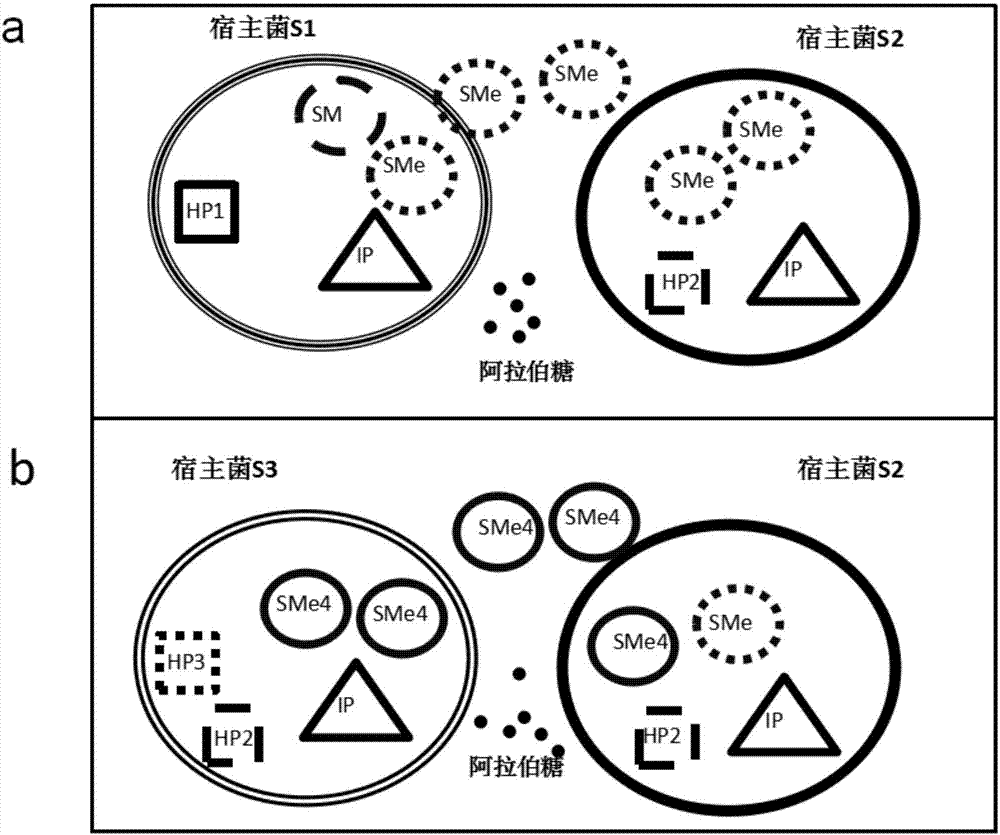
[0068] The preparation of the host bacteria required for the evolution of embodiment 1
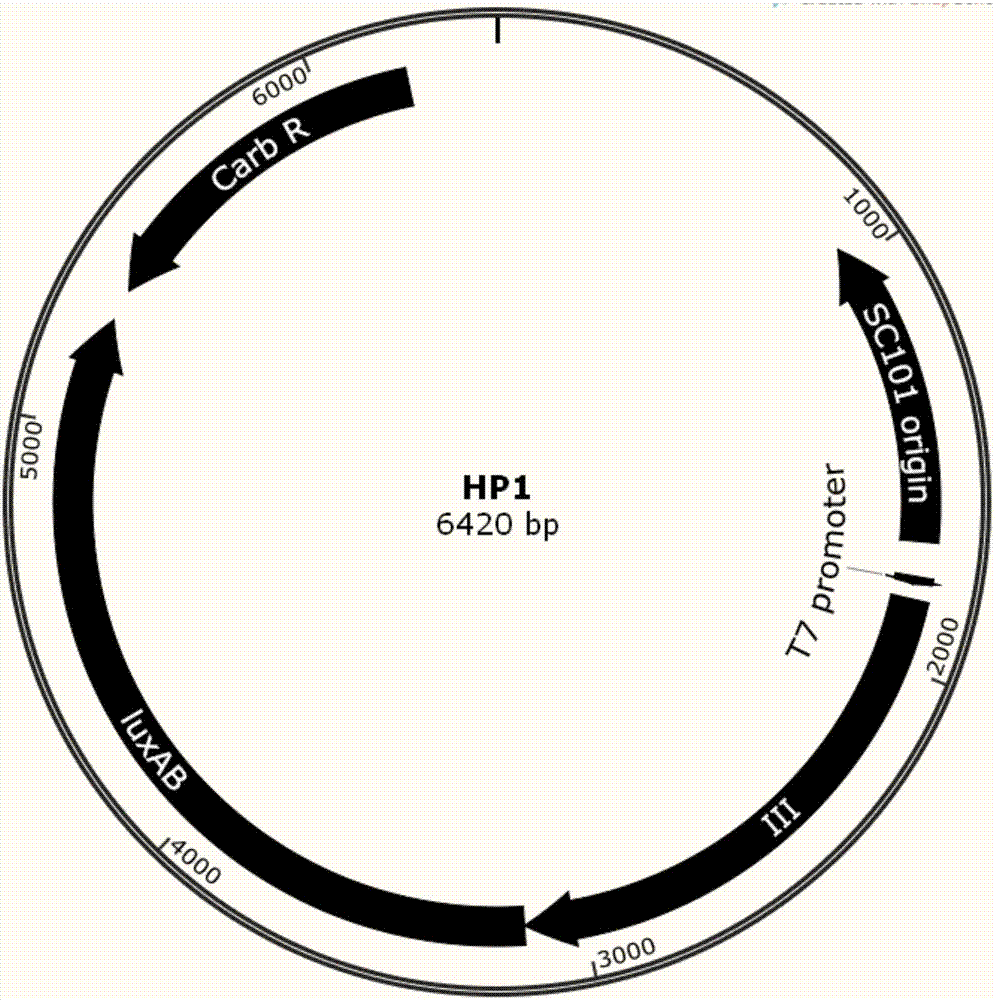
[0069] 1) The host strain S1 carrying the HP1 and IP plasmids was cultured in LB medium containing 50 μg / ml carbenicillin and 25 μg / ml chloramphenicol resistance at 37° C. and 220 rpm until OD600 = 0.6. Store the bacterial solution at 4°C for later use, and the storage time should not exceed 1 day.
[0070] 2) The host strain S2 carrying the HP2 and IP plasmids was cultured in LB medium containing 50 μg / ml carbenicillin and 25 μg / ml chloramphenicol resistance at 37° C. and 220 rpm until OD600=0.6. Store the bacterial solution at 4°C for later use, and the storage time should not exceed 1 day.
[0071] 3) The host bacteria S3 carrying the HP2, HP3 and IP plasmids were placed in the LB medium containing 50 μg / ml carbenicillin, 50 μg / ml spectinomycin and 25 μg / ml chloramphenicol resistance, at 37 ° C, 220 rpm Cultivate to OD600=0.6. Centrifuge at 12000 rpm for 1 min to collect the bacteria,...
Embodiment 2
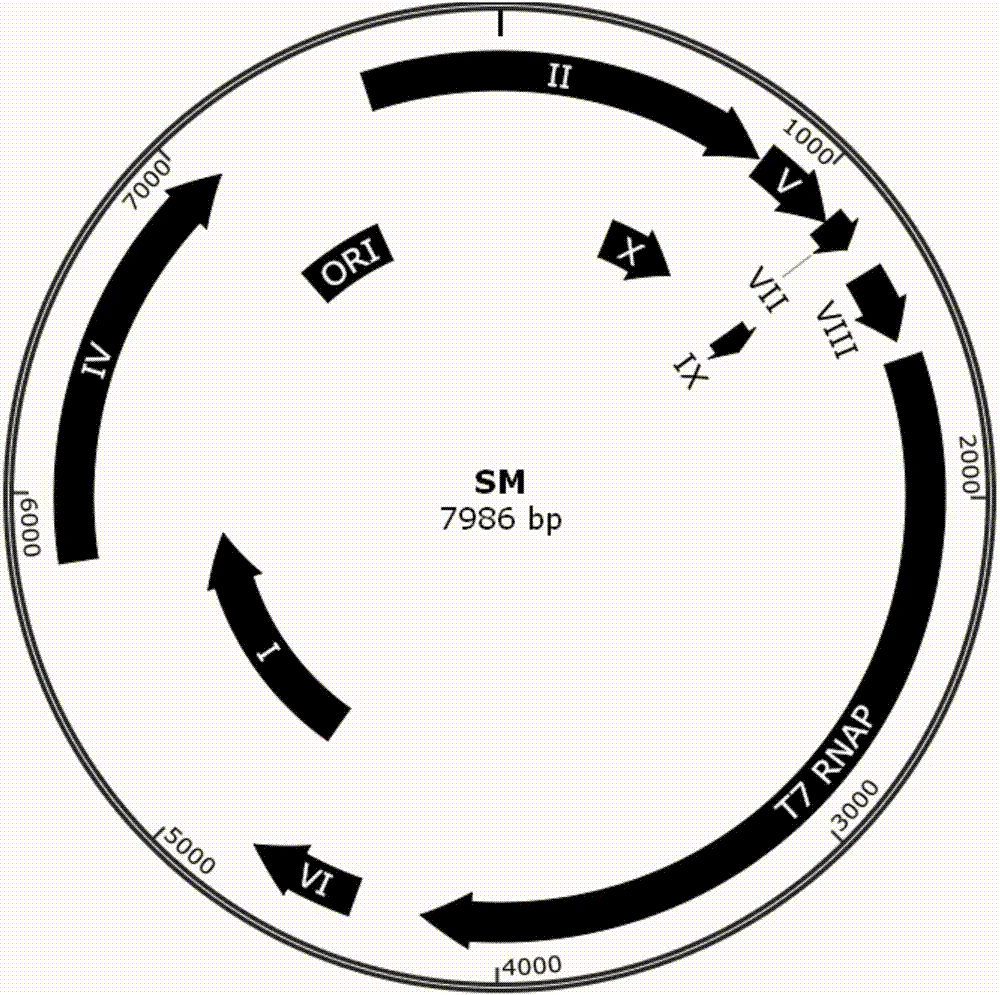
[0077] SM phage plaque observation method in embodiment 2S5 host bacterium
[0078] 1) Spread a layer of 10ml of 2% agarose gel on a 10cm bacterial culture plate, and let it solidify at room temperature for 20min.
[0079] 2) When the concentration of SM is unknown, it is necessary to divide SM by 0 times and 10 1,2,3,4,5 Doubling serial dilutions were performed.
[0080]3) Take 6 groups of 200 μL of the S5 host bacteria prepared in Example 1, add 10 μL of SM with different dilution gradients in “2)” to each group, and then add 4 ml of LB medium containing 0.4% agar stored at 55° C. and the final Concentration 50 μg / ml carbenicillin. After mixing on a vortex mixer, the samples were spread onto the plates prepared in "1)".
[0081] 4) Cultivate overnight in a biochemical incubator at 37°C.
[0082] 5) Observing and counting the number of plaques formed by each serially diluted sample, and calculating the SM concentration.
Embodiment 3
[0083] SM phage plaque observation method in embodiment 3S6 and S7 host bacteria
[0084] Same as in Example 2, it is sufficient to replace the host bacteria forming plaques with the S6 bacteria and S7 bacteria prepared in Example 1, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com