Method for improving dissolution rate of ezetimibe tablet
A technology of ezetimibe and dissolution rate, which is applied in pill delivery, metabolic diseases, drug combination, etc., can solve problems such as difficult process requirements, unsuitable for industrial production, high price, etc., and achieves high safety, easy process scale-up, The effect of request reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
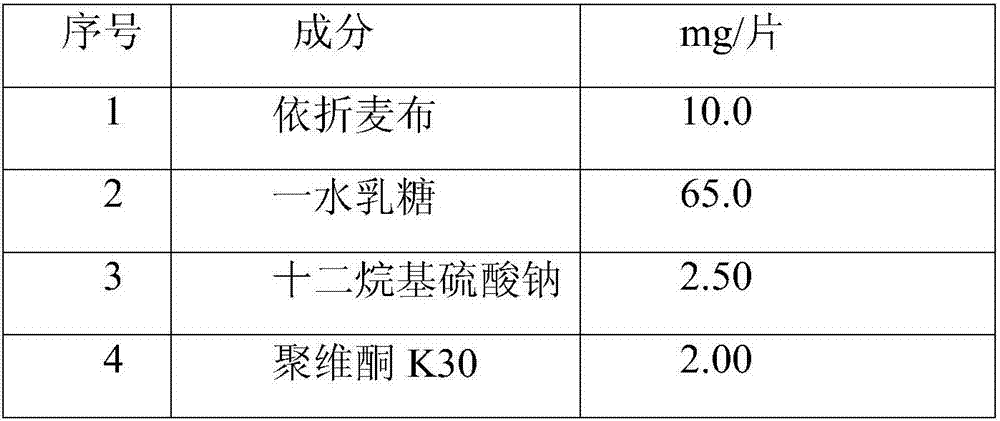
[0059] 1. Preparation prescription:
[0060] The bulk drug obtained by common jet milling.
[0061] serial number
Element
mg / tablet
1
10.0
2
lactose monohydrate
55.0
3
2.00
4
Povidone K30
4.00
5
8.00
6
20.0
7
1.00
total
100
[0062] 2. Preparation process
[0063] The bulk drug obtained by common jet milling. In wet granulation, ingredients 1, 2, 3, 4 and part No. 5 are mixed, purified water and ethanol are prepared into a 50% ethanol solution as a wetting agent, and the weight ratio of the wetting agent to ezetimibe is 0.7:1, that is 7mg. The wetting agent is sprayed into the mixture and granulated and dried. The dry granules are sieved and mixed with the remaining ingredients No. 5 and No. 6. Add ingredient No. 7 and mix. The mixture is compressed...
Embodiment 2
[0065] 1. Preparation prescription: same as Example 1.
[0066] The bulk drug obtained by common jet milling.
[0067] 2. Preparation process:
[0068] The bulk drug obtained by common jet milling. Mix No. 1, 2, 3 and part No. 5 components in a fluidized bed, mix No. 4 component with 50% ethanol solution to prepare an adhesive, and the weight of ethanol aqueous solution is 10 times that of No. 4 component, ie 40mg. The binder is sprayed into the mixture and granulated and dried. The dry granules are sieved and mixed with the remaining ingredients No. 5 and No. 6. Add ingredient No. 7 and mix. The mixture is compressed to the appropriate size and weight on a suitable tablet machine.
Embodiment 3
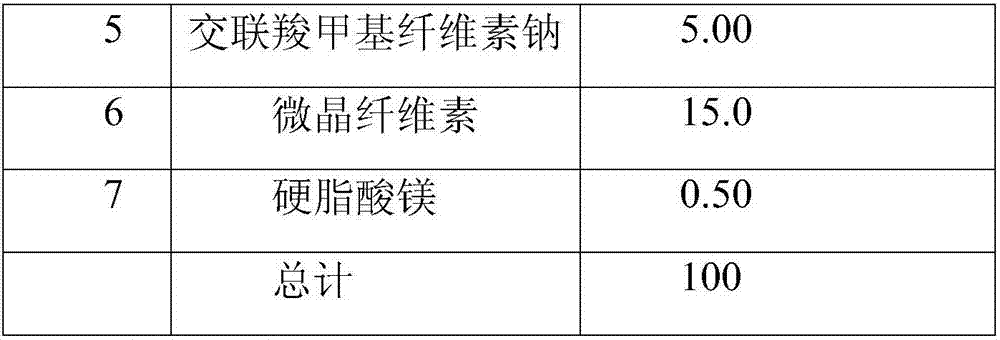
[0070] 1. Preparation prescription:
[0071] The bulk drug obtained by common jet milling.
[0072] serial number
Element
mg / tablet
1
Ezetimibe
10.0
2
lactose monohydrate
40.0
3
1.00
4
Povidone K30
8.00
5
15.00
6
25.0
7
1.00
total
100
[0073] 2. Preparation process:
[0074] The bulk drug obtained by common jet milling. Mix No. 1, 2, 3 and part No. 5 components in a fluidized bed, mix No. 4 component with 50% ethanol solution to prepare an adhesive, and the weight of the ethanol aqueous solution is 10 times that of No. 4 component, ie 80mg. The binder is sprayed into the mixture and granulated and dried. The dry granules are sieved and mixed with the remaining ingredients No. 5 and No. 6. Add ingredient No. 7 and mix. The mixture is compressed to the appropri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com